Abstract
Adherent epithelial cells require interactions with the extracellular matrix for their survival, though the mechanism is ill-defined. In long term cultures of primary mammary epithelial cells, a laminin-rich basement membrane (BM) but not collagen I suppresses apoptosis, indicating that adhesion survival signals are specific in their response (Pullan et al. 1996. J. Cell Sci. 109:631–642). We now demonstrate that the signal from BM is mediated by integrins and requires both the α6 and β1 subunits. In addition, a hormonal signal from insulin or insulin-like growth factors, but not hydrocortisone or prolactin, is necessary to suppress mammary cell apoptosis, indicating that BM and soluble factors cooperate in survival signaling. Insulin induced autophosphorylation of its receptor whether mammary cells were cultured on collagen I or BM substrata. However, both the tyrosine phosphorylation of insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase were enhanced in cells cultured on BM, as was the phosphorylation of the phosphatidylinositol 3-kinase effector, protein kinase B. These results suggest a novel extracellular matrix–dependent restriction point in insulin signaling in mammary epithelial cells. The proximal signal transduction event of insulin receptor phosphorylation is not dependent on extracellular matrix, but the activation of downstream effectors requires adhesion to BM. Since phosphatidylinositol 3-kinase was required for mammary epithelial cell survival, we propose that a possible mechanism for BM-mediated suppression of apoptosis is through its facilitative effects on insulin signaling.
Keywords: extracellular matrix, mammary gland, apoptosis, integrin, insulin
Cells in multicellular organisms monitor their environment through interactions with neighboring cells and through receptors for both soluble and locally acting factors including extracellular matrix (ECM)1. In addition to providing signals for proliferation, differentiation, and migration, such interactions are also essential for survival and in their absence, cells undergo apoptosis. While the cellular signaling pathways that control the decision to enter apoptosis are currently being studied extensively, little is known about the interplay between survival factors derived from the soluble milieu and the ECM.
The mammary gland is a particularly useful system to study mechanisms that regulate apoptosis. Most of its epithelial cells are removed by apoptosis during involution of the gland after lactation, and the survival of purified mammary epithelial cells can be manipulated in primary culture (Strange et al., 1992; Pullan et al., 1996). Several models have recently emerged to explain the post-lactational induction of apoptosis. One possibility is that cessation of suckling results in hormonal changes in the nursing mother that alter the supply of soluble cell survival factors. As an example of this, withdrawal of soluble survival factors such as insulin or insulin-like growth factor I (IGF-I) from cultured cells results in apoptosis (Rodriguez-Tarduchy et al., 1992; Harrington et al., 1994; Parrizas et al., 1997). In the mammary gland in vivo, the expression of an IGF-binding protein which inhibits IGF action, IGFBP-5, is dramatically increased after weaning and may therefore act to trigger apoptosis (Tonner et al., 1997). Alternatively, expression of matrix-degrading metalloproteinases after weaning may trigger cell death by breaking down cell–ECM interactions (Talhouk et al., 1992; Alexander et al., 1996; Lund et al., 1996). Although these hypotheses offer plausible explanations for the induction of apoptosis in vivo, none have been proven, suggesting that the mechanism may be more easily deciphered using a cell culture model. However, even in cell cultures, very little is currently known about the control of mammary epithelial apoptosis.
Epithelial cells undergo apoptosis if they are denied contact with ECM (Frisch and Francis, 1994; Khwaja et al., 1997) and indeed, ECM is a survival factor in a number of cell types (Frisch and Ruoslahti, 1997). The ECM is linked to the cytoskeleton and to cytoplasmic signaling pathways through integrins, and both integrins and some of their downstream effectors, including p125FAK, are involved in the survival response (Frisch et al., 1996). However, there are very few natural situations where simple epithelial cells are denied contact with the ECM for appreciable periods of time, and we have therefore developed a more physiological model than previously used to examine ECM survival signaling. Primary cultures of mouse mammary epithelial cells and mammary cell lines show a differential long term apoptotic response to different ECM in tissue culture (Boudreau et al., 1995; Pullan et al., 1996). On collagen I–coated substrata these cells form organized cobblestone monolayers, express E-cadherin and desmocollins at their cell–cell junctions, and are polarized (Streuli, 1995; Runswick et al., 1996). However, when cultured on specialized ECM known as basement membrane (BM), which contains laminin, collagen IV, nidogen and perlecan, they develop into three-dimensional structures which mimic alveoli in vivo (Aggeler et al., 1991). Although in both situations they interact with the respective substrata through integrins, cells cultured on collagen I show extensive apoptosis over periods of several days whereas those in contact with BM do not (Pullan et al., 1996). Since mammary cells interact with collagen and BM through different integrin receptors, it is likely that only certain integrins deliver a survival signal (Howlett et al., 1995; Metcalfe and Streuli, 1997).
Recent models for integrin-mediated control of phenotype in adherent cells have indicated that integrins might cooperate with soluble factors to drive signaling pathways, possibly through the formation of multi-protein adhesion complexes (Miyamoto et al., 1996; Schneller et al., 1997). Indeed, both proliferation and differentiation (Streuli et al., 1995; Zhu et al., 1996) are dependent on ECM as well as growth and differentiation factors. For example, cell anchorage regulates the activation of mitogen activated protein kinase by serum or EGF, and epithelial cell interactions with BM are necessary for prolactin to trigger the kinase Jak2 and activate the transcription factor Stat5 (Lin et al., 1997; Renshaw et al., 1997; Edwards et al., 1998).
In this paper we have sought to determine whether soluble factors and BM act coordinately to regulate the suppression of apoptosis in primary cultures of mammary epithelium. We show that insulin is an essential survival ligand but that the cell–ECM interactions that lead to survival are necessary for insulin to trigger its downstream survival signaling pathway.
Materials and Methods
Cell Culture
Primary mammary epithelial cells were prepared from 14.5–18.5-d pregnant ICR mice as previously described (Pullan et al., 1996). After culture for 3 days, cells were trypsinized, passed through a 70-μm nylon mesh (Falcon) to remove large cell aggregates, and plated at 1 × 105 cells/cm2 in DMEM/F12 medium containing 5% fetal calf serum (Advanced Protein Products) and 5 ng/ml epidermal growth factor (Harlan Sera-Lab) overnight, then washed and cultured in serum-free DMEM/F12 medium containing appropriate combinations of 2.8 nM hydrocortisone, 880 nM insulin, 150 nM prolactin (Sigma), with or without inhibitors of the insulin signaling pathway. Some experiments included IGF-I or IGF-II (R&D systems) in place of insulin.
The ECM substrata used for these studies were rat tail collagen I and reconstituted BM matrix prepared from Engelbreth-Holm-Swarm (EHS) tumor (Pullan and Streuli, 1996). Collagen I was coated onto tissue culture plates overnight at 80 μg/ml and washed extensively before use, or in some experiments commercially precoated Biocoat dishes were used (Becton Dickinson). A factor-reduced preparation of the BM matrix was prepared using sequential ammonium sulfate precipitations (Taub et al., 1990) and coated onto tissue culture plastic at 14 mg/ml. In some experiments, factor-reduced BM matrix was diluted into the culture medium (final concentration 100 or 400 μg/ml) and overlaid onto first passage cells plated on collagen I (Streuli et al., 1995).
Apoptosis Assays
To analyze DNA integrity, first passage cells were cultured in medium containing appropriate hormones and inhibitors for two days, then washed over a period of 2 h to remove the accumulated dead cells and debris. Fresh medium was added to cells and after 4 h newly apoptotic cells were collected from the medium and pooled with any remaining attached cells. DNA was extracted from samples (Pullan et al., 1996), its OD was measured at 260 nm, and separated on a 1% agarose gel to confirm DNA loading after staining the gel with ethidium bromide. Equal amounts of DNA were separated by conventional agarose gel electrophoresis, southern blotted, and apoptotic DNA ladders were visualized by hybridization with a digoxigenin-labeled total mouse genomic DNA probe (Boehringer Mannheim).
Single cell cultures were prepared by straining first passage cells through a 20-μm mesh, pelleting and resuspending in factor-reduced BM matrix at 2–4 × 106 cells/ml, as described (Streuli et al., 1991). 100 μl of the mammary cell suspension was gelled for 60 min on dishes precoated with a thin layer of BM, then cultured in serum-free DMEM/F12 medium containing appropriate hormones, inhibitors or antibodies for 2 d before fixation in 2% paraformaldehyde. 25-μm cryosections were stained with 0.5 μg/ml Hoechst 33258 and apoptotic nuclei were counted in the single cell population. Each experiment was repeated two to three times and in each experimental condition, >600 single cells were scored for apoptosis. Polyclonal rabbit anti–β1 integrin antibody was prepared in this laboratory (Edwards and Streuli, 1998). It was shown to inhibit the adhesion of first passage mouse mammary epithelial cells to laminin, collagen IV, but not fibronectin or vitronectin. Purified rat monoclonal antibody to α6 integrin (GoH3) and its rat IgG2a isotype control were obtained from Serotec. Rabbit polyclonal anti-laminin antibody was raised against purified laminin isolated from EHS tumor (a kind gift of P. Yurchenco, Robert Wood Johnson Medical School, Piscataway, NJ). This laminin had previously been shown to contain laminin A and B chains but not collagen IV or nidogen (Streuli et al., 1995). A protein A–derived IgG fraction from the rabbit serum was subsequently purified on an EHS affinity column. This antibody significantly inhibited the adhesion of first passage mouse mammary epithelial cells to laminin at 5 μg/ml and completely inhibited adhesion at 30 μg/ml (data not shown).
A quantitative assay for apoptosis based on measuring cellular detachment from different substrata was previously described (Pullan et al., 1996). Cells detaching into the medium of first passage cultures over periods of 4 h were pelleted and counted using either a hemocytometer or a Coulter counter. Detached cells were confirmed to have apoptotic morphology by fluorescence microscopy after staining with 0.5 μg/ml Hoechst 33258, and the remaining monolayers were also stained with Hoechst 33258 to confirm that apoptotic cells did not accumulate there. Quantitative data for detachment assays are all relative to the number of cells that attached to each substrata after plating.
Protein Analysis
Mammary cells were cultured for 2 d, then washed and serum-free DMEM/F12 medium with or without insulin was replaced for 15 min. The cells were washed twice with 1 mM Na3VO4, 10 mM NaF in PBS and scraped into NET lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 1% NP-40, 2 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 10 μg/ml aprotinin, 20 μM leupeptin, 1 mM PMSF, pH 7.5). Cells were lysed by vortexing and then rotating for 30–60 min at 4°C, and detergent insoluble proteins were cleared by centrifugation at 7,000 g. Aliquots of samples were subjected to SDS-PAGE on 6% gels, and the gels were stained with Coomassie Brilliant Blue to normalize protein levels. Equal amounts of protein were immunoprecipitated either with anti–IRS-1 antibody (UBI) or anti-insulin receptor β antibody (Transduction Laboratories) and 20 μl protein A–Sepharose beads (Zymed Laboratories), before being separated on 6% gels under reducing conditions and transferred to Immobilon-P membranes (Millipore). Phosphotyrosine blots were blocked in 1% BSA in TBST, (50 mM Tris-Cl, 150 mM NaCl, 0.1% Tween 20, pH 7.5) and incubated overnight with monoclonal antibody 4G10 (UBI) at 1:1,000 for insulin receptor β or 1:3,000 for IRS-1. Interaction of the p85 subunit of PI 3-kinase with IRS-1 was examined by probing IRS-1 immunoblots with anti–PI 3-kinase (UBI). For each experiment, equal amounts of protein were confirmed for IRS-1 by blocking IRS-1 immunoblots in 3% nonfat dry milk/PBS and probing with 1:3,000 anti–IRS-1 antibody; for insulin receptor β, whole cell lysate blots were blocked with 3% BSA/TBST and probed with 1:250 anti-insulin receptor β. Protein kinase B (PKB, otherwise known as c-Akt) was analyzed by immunoblotting equal amounts of cell protein with either an anti-PKB antibody or an antibody specific for phosphorylated PKB (both at 1:2,000; New England Biolabs). Proteins were visualized by enhanced chemiluminescence (Amersham).
Results
Survival of Mammary Epithelium Is Regulated Coordinately by Insulin and BM
Mammary epithelial cells require appropriate cell–ECM interactions for survival since they undergo apoptosis in the absence of a BM (Pullan et al., 1996). To determine the mechanism by which BM suppresses apoptosis, we asked whether BM was able to act as a survival ligand by itself or whether additional factors were required to prevent cell death. First passage mammary epithelial cells isolated from midpregnant mice were plated on substrata of collagen I or a reconstituted BM matrix derived from the EHS tumor. Culture on a BM matrix in the presence of lactogenic hormones, insulin, prolactin, and hydrocortisone, suppressed apoptosis, whereas cells died on collagen I (Fig. 1 A). However, removal of lactogenic hormones from the medium resulted in apoptotic death of the cells, even those cultured on the BM matrix (Fig. 1 A). This indicated that survival of mammary cells required two signals, one from the BM and a second signal from the hormonal milieu.
Figure 1.
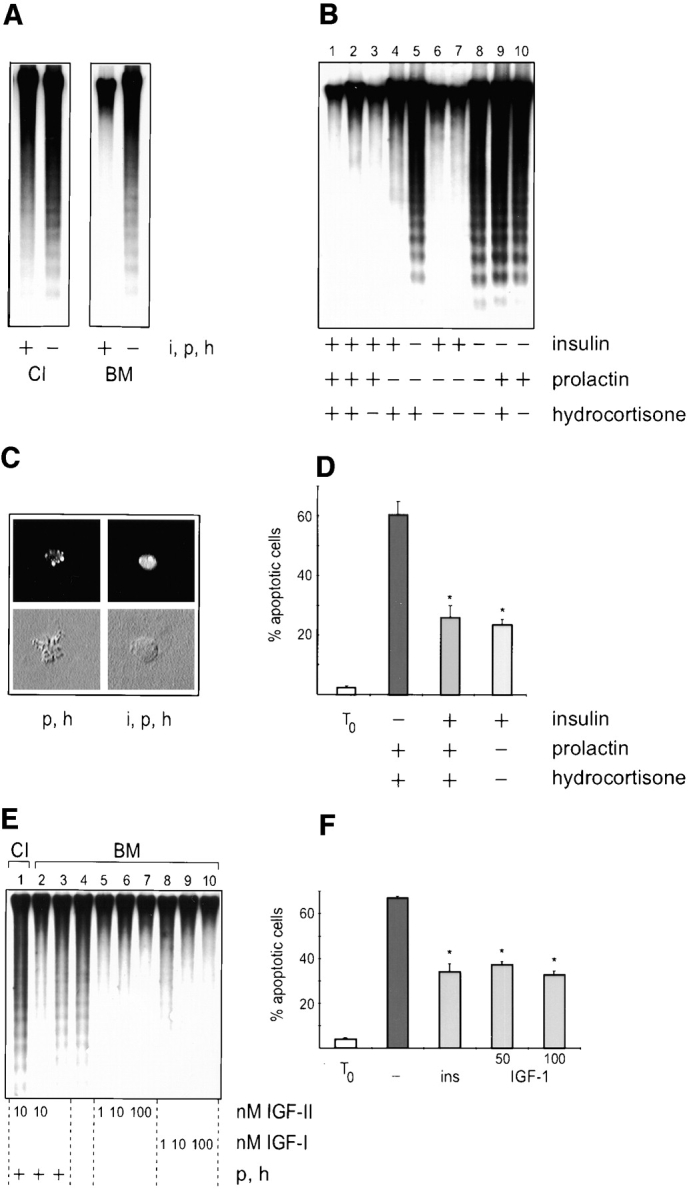
Coordinate signaling between BM and insulin regulates mammary survival. (A) Southern analysis of DNA isolated from cells cultured on collagen I (CI) or BM in the presence (+) or absence (−) of insulin, prolactin and hydrocortisone (i, p, and h). Note that DNA fragmentation is only suppressed in cells cultured on BM together with lactogenic hormones. (B) Southern analysis of DNA isolated from cells cultured on BM with combinations of insulin, prolactin and hydrocortisone. Samples in lanes 2 and 7 were from cells cultured in phenol red–free media, and the results indicate that estrogenic activity of phenol red did not contribute to survival. (C) Cryosections of cells suspended within gels of BM matrix after Hoechst staining (upper panels) and the corresponding differential interference contrast images (lower panels). These images are typical of apoptotic single cells cultured with hydrocortisone and prolactin (p and h), and those which resisted apoptosis when cultured additionally with insulin (i, p, and h). (D) Quantitative analysis of apoptosis in single cells embedded within BM. The percentage of apoptotic cells, as determined by nuclear morphology after Hoechst staining, is shown in cultures incubated with combinations of insulin, prolactin and hydrocortisone. Results are the mean ± SE of counts from five independent cultures from two separate experiments. The values with insulin are significantly different to those without insulin (*P < 0.01). Less than 5% of the cells were apoptotic in embedded cultures that were fixed 1 h after plating (To). (E) Southern analysis of DNA isolated from cells cultured on collagen I or BM in the presence of 1, 10, or 100 nM IGF-I or IGF-II. Lanes 1–3 additionally contained prolactin and hydrocortisone (p and h). (F) Quantitative analysis of IGF-I suppression of apoptosis in single cells embedded within BM. Results are the mean ± SE of counts from six independent cultures from two separate experiments. The values with insulin and IGF-I are significantly different to those without hormones (*P < 0.05). Less than 5% of the cells were apoptotic at the time of plating (To).
To determine which of the three hormones acted in a survival capacity, cells plated on BM were incubated with combinations of insulin, prolactin, and hydrocortisone and the extent of cell death was measured by DNA fragmentation. Apoptosis occurred only when the cells were cultured without insulin (Fig. 1 B). We also used an alternate assay for apoptosis, where single mammary cells were cultured within a BM gel, but apart from each other so that they were unable to form cell–cell interactions (Streuli et al., 1991). In this assay, apoptosis was measured by determining nuclear morphology after culture for 48 h (Fig. 1 C). 20–30% of single cells were apoptotic providing insulin was present in the medium. However, in the absence of insulin, cell death increased significantly (P < 0.01) to 60– 70% (Fig. 1 D). These two different approaches collectively demonstrate that BM does not act alone to suppress apoptosis in primary cultures of mammary epithelia. Instead, it regulates survival in combination with signals elicited by insulin. Furthermore, the data show survival is not dependent on mammary differentiation, since removal of prolactin and hydrocortisone, which are required for milk protein gene expression, did not result in apoptosis.
In addition to the ability of insulin to interact with its own receptor, this hormone can also bind to the IGF receptor although with a 100–1,000-fold lower affinity (Parrizas et al., 1997). IGF-I or IGF-II have been shown to act as survival factors in other cell types (Rodriguez-Tarduchy et al., 1992; Harrington et al., 1994; Kulik et al., 1997), and we therefore tested whether they could directly suppress mammary apoptosis. At physiological concentrations, both IGF-I and IGF-II significantly inhibited the DNA fragmentation exhibited by cells cultured on a BM matrix in the absence of other hormones (Fig. 1 E). Moreover, IGF-I suppressed apoptosis (P < 0.01) in single mammary cells cultured within the BM gel to the same extent as insulin (Fig. 1 F). The survival of mammary epithelia can therefore be regulated by signals from IGF-I and IGF-II in addition to those from insulin, and these signals act coordinately with BM.
Laminin Is a Survival Ligand for Mammary Epithelial Cells
Mammary epithelial cells plated onto a BM substratum form multicellular, alveolar-like structures (Barcellos-Hoff et al., 1989; Aggeler et al., 1991). Thus, it was important to ask whether this complex three-dimensional structure was involved with mammary cell survival, or if signals from the BM–integrin interactions were sufficient. Therefore, we cultured mammary cells as monolayers on collagen I and incubated them with the EHS BM preparation diluted into the culture medium. Under these conditions, the cells did not form alveoli but remained as monolayers on the culture dish with some matrix proteins precipitating over the cells (Streuli et al., 1995). Dilution of the BM preparation 140-fold to 0.1 mg/ml resulted in significant suppression of apoptosis, both in a quantitative assay (Fig. 2 A) and in DNA fragmentation studies (Fig. 2 B). BM proteins diluted 35-fold to 0.4 mg/ml suppressed apoptosis to virtually the same extent as in the cells cultured as alveoli on top of a BM substratum (Fig. 2, A and B). Thus, the three-dimensional multicellular structure is not a primary determinant of mammary cell survival.
Figure 2.
Inhibition of mammary epithelial apoptosis by BM is not dependent on three-dimensional multicellular structure. Mammary cells cultured as monolayers on collagen I (0 mg/ml EHS) were incubated with lactogenic hormones and the indicated concentrations of BM proteins diluted into the culture medium (0.1, 0.4 mg/ml EHS), or as alveoli on top of BM (14 mg/ml EHS). (A) Apoptosis was measured in a quantitative assay. Each day during a 3-d experiment, the cultures were washed and then the detached apoptotic cells were collected over a 4-h time period. Apoptosis is expressed as the number of apoptotic cells per 1,000 cells initially attached to the culture dish. Results are the mean ± SE of two separate experiments. The number of cells that died toward the end of the experiment was lower than on day 1 because at this time there were fewer cells remaining on the dish. (B) Apoptosis was measured by DNA fragmentation analysis as in Fig. 1 A.
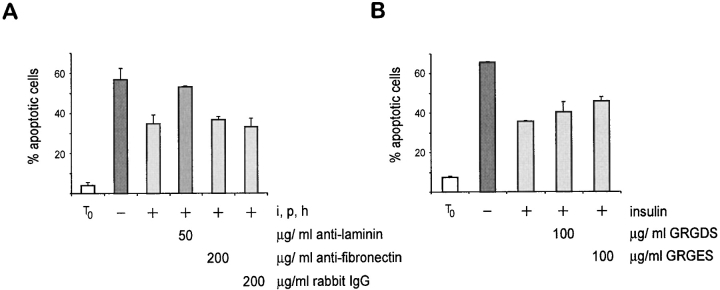
BM contains several ECM proteins and one component, laminin, has previously been shown to be a survival ligand for other cell types (Kim et al., 1994; Vachon et al., 1996; Bozzo et al., 1997). To determine whether laminin was a survival factor for mammary epithelial cells we developed a function-blocking anti-laminin antibody that inhibited adhesion of mammary cells to laminin (data not shown). We assessed whether this antibody was able to interfere with the survival of mammary cells that had been cultured as single cells within BM gels (Fig. 3 A). We found that when the anti-laminin antibody was included in the gel, mammary cells underwent apoptosis even when they were cultured in the presence of insulin. In contrast, an adhesion-blocking antibody directed against fibronectin had no effect (Fig. 3 A), neither did the inclusion of an RGD peptide in the assay (Fig. 3 B). These results demonstrated that within the context of a three-dimensional BM gel, laminin has a survival-promoting function. Indeed, they show that both insulin and laminin can act as survival ligands for mammary epithelial cells, although they do not rule out the possibility that other soluble factors or ECM proteins may have a similar function.
Figure 3.
Laminin affects the survival potential of mammary epithelial cells. (A) Quantitative analysis of apoptosis in single cells embedded within BM in the presence of anti-laminin and fibronectin antibodies. The percentage of apoptotic cells, as determined by nuclear morphology after Hoechst staining, is shown. Results are the mean ± SE of counts from four independent cultures from two separate experiments. The values with anti-laminin antibody are significantly different to those for control IgG and anti-fibronectin antibody (P < 0.001). Note that insulin together with prolactin and hydrocortisone failed to suppress apoptosis in mammary cells cultured with anti-laminin antibody. (B) Quantitative analysis of apoptosis in single cells embedded within BM in the presence of GRGDS and its control GRGES peptides. Note that there was no significant different difference in levels of apoptosis between those cells cultured with and without GRGDS (P = 0.34).
BM Regulates Mammary Survival through α6 and β1 Integrin Subunit
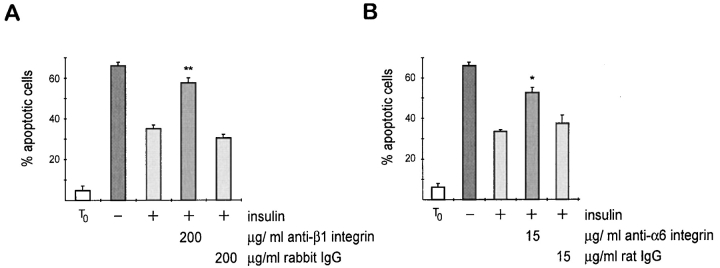
Major cellular receptors for BM proteins, including laminin, are the transmembrane heterodimeric integrin receptors. To confirm that BM acts directly to suppress apoptosis, and that the survival signals were mediated by integrins, we assessed whether function-blocking anti-integrin antibodies interfered with the mammary cell survival using the single cell assay. In experiments where an adhesion-blocking polyclonal anti–mouse β1 integrin antibody was included in the gel, insulin failed to suppress apoptosis. Indeed, the levels of death were similar to those observed in single mammary cells in the absence of insulin (Fig. 4 A). One receptor for laminin is α6β1 integrin, and we therefore performed parallel experiments using a function-blocking anti–α6 integrin antibody (Fig. 4 B). The cellular response was similar to that for the anti–β1 integrin antibody, providing evidence that both the α6 and β1 integrin subunits can transduce survival signals in normal mammary epithelial cells. Suitable antibodies to other mouse integrin subunits were not available to test for specificity. Therefore, we performed similar experiments using function-blocking anti–human integrin antibodies and demonstrated that α6 and β1 integrin, but not α2 integrin, acts as survival receptors in primary cultures of human mammary cells (Oliver, J., M. O'Hare, and C.H. Streuli, manuscript in preparation). In addition, our results with anti-fibronectin antibodies and RGD peptides do not support a role for αv integrins in mammary cell survival.
Figure 4.
Requirement of integrin for mammary cell survival. Quantitative analysis of apoptosis in single cells embedded within BM in the presence of anti-integrin antibodies. The percentage of apoptotic cells, as determined by nuclear morphology after Hoechst staining, is shown. Results are the mean ± SE of counts from five independent cultures from two separate experiments. The values with anti-integrin antibody are significantly different to those for control IgG (*P < 0.05; **P < 0.01). Insulin failed to suppress apoptosis in mammary cells cultured with either (A) anti– β1 integrin or (B) anti–α6 integrin antibody.
Our combined data using function-blocking antibodies to laminin and to integrin subunits show that suppression of apoptosis in mammary epithelia depends on interactions of the cells with BM through the α6 and/or β1 integrin receptors, and that laminin can act as a survival ligand.
BM and Insulin Regulate Survival in Mammary Cells by Cross Talk of Signaling Pathways
Since both insulin and laminin had been identified as mammary cell survival factors, it is possible that they trigger separate pathways required for suppressing apoptosis. However, recent work on the control of cell cycle and differentiation indicates that ECM affects the ability of growth factors/cytokines to trigger their downstream signaling kinases (Lin et al., 1997; Renshaw et al., 1997; Edwards et al., 1998). Thus an alternative mechanism to explain survival signaling in mammary cells is that the insulin- and BM-triggered pathways converge. To determine whether this was indeed the case, the extent of tyrosine phosphorylation in the proximal components of the insulin signaling pathway was measured (Fig. 5).
Figure 5.


Cross talk between insulin and BM signaling pathways. Mammary cells cultured on either collagen I or BM were stimulated for 15 min with insulin or left unstimulated. (A) Cell lysates were immunoprecipitated with anti-insulin receptor β (Ins-Rβ) antibody and precipitated proteins were analyzed by immunoblotting for phosphotyrosine (4G10; top). Equivalent amounts of insulin receptor β were confirmed by SDS-PAGE of the same amount of whole cell lysate followed by immunoblotting with anti-insulin receptor β antibody (bottom). (B) Duplicate cell lysates were immunoprecipitated with anti–IRS-1 antibody and precipitated proteins were analyzed by immunoblotting for either phosphotyrosine using 4G10 (top) or for IRS-1 itself to confirm that equal levels of IRS-1 were present in all the samples (bottom). (C) Association of PI 3-kinase with IRS-1 was determined by immunoprecipitating lysates with anti–IRS-1 antibody, followed by immunoblotting with antibodies to either phosphotyrosine, the p85 subunit of PI 3-kinase (p85), or the precipitating antibody. (D) The signal from the experiment in C was quantitated by scanning densitometry and the level of phosphotyrosine or PI 3-kinase in each sample was normalized to the level of IRS-1. Note that threefold more PI 3-kinase associated with IRS-1 in cells cultured on BM than in cells cultured on collagen I.
Insulin interacts with its receptor, causing receptor oligomerization and activation of the kinase domain. This results in tyrosine phosphorylation of the receptor β subunit. The adaptor protein insulin receptor substrate-1 (IRS-1) is recruited to the insulin receptor, then becomes tyrosine phosphorylated creating SH2 domain interaction sites for downstream enzymes such as PI 3-kinase (Myers et al., 1994; Sun et al., 1992). The ability of insulin to signal in primary cultures of mammary cells plated on BM was compared with those on collagen I. Tyrosine phosphorylation of the insulin receptor β subunit occurred within 15 min of insulin treatment and was independent of cell– ECM interactions (Fig. 5 A). However, the ability of IRS-1 to become tyrosine phosphorylated in response to insulin was strongly dependent on cell interactions with BM, and virtually no IRS-1 phosphorylation was observed in cells cultured on collagen I (Fig. 5 B).
It has been shown in several cell types that PI 3-kinase is required for preventing apoptosis, and that in neuronal cells and fibroblasts IGF-I suppresses apoptosis through PI 3-kinase (Yao and Cooper, 1995; Minshall et al., 1996; Dudek et al., 1997; Kennedy et al., 1997; Kulik et al., 1997; Miller et al., 1997; Parrizas et al., 1997). PI 3-kinase is activated in response to insulin or IGF-I following the interaction of its p85 subunit with IRS-1, and therefore we assessed whether cell–ECM interactions regulated the association of the p85 subunit of PI 3-kinase with IRS-1 (Myers et al., 1994). PI 3-kinase did not associate with IRS-1 in mammary cells cultured on either substratum in the absence of insulin (Fig. 5 C). However, within 15 min of insulin treatment PI 3-kinase bound IRS-1, but the extent of the interaction was strongly dependent on cell adhesion to BM; quantitation of the data indicated that threefold more PI 3-kinase bound to IRS-1 in cells cultured on a BM substratum than in cells cultured on collagen I (Fig. 5 D).
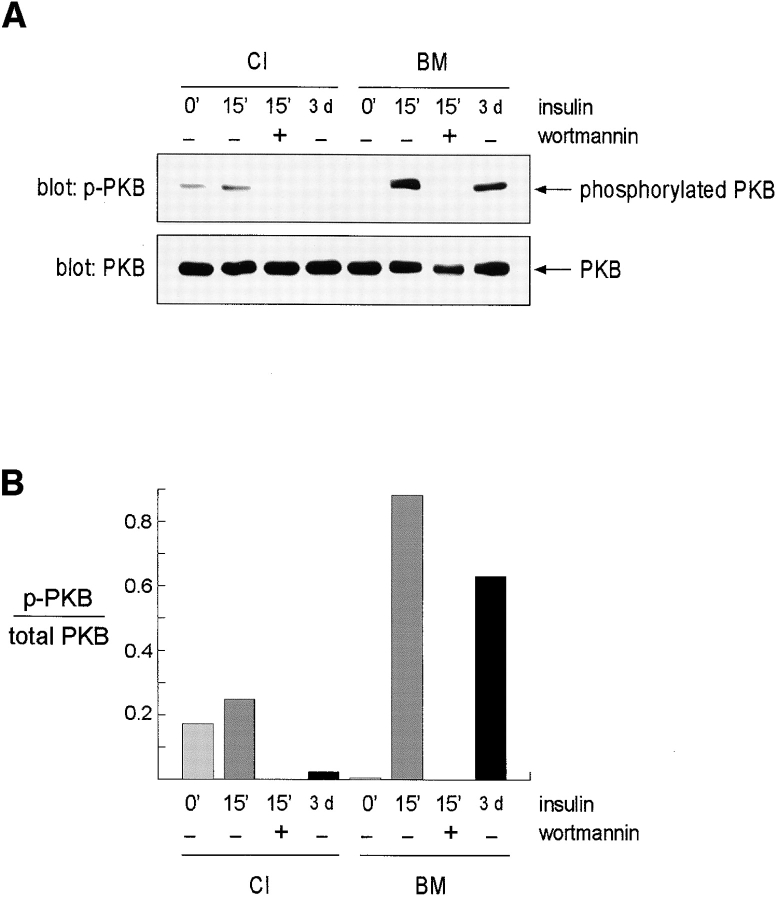
One downstream target of PI 3-kinase signaling pathway is PKB, which has previously been implicated in the suppression of apoptosis (Dudek et al., 1997; Kauffmann-Zeh et al., 1997; Kulik et al., 1997; Crowder and Freeman, 1998). This enzyme is recruited to the plasma membrane following PI 3-kinase phosphorylation of membrane lipids, and then activated phosphorylation on serine/threonine residues (Downward, 1998). Using an antibody specific for phosphorylated PKB, we found that maximal activation only occurred when mammary cells treated with insulin had been cultured on BM (Fig. 6). Wortmannin completely abrogated insulin-induced PKB phosphorylation. Since many of the assays to measure mammary apoptosis in our work were carried out over several days, we also measured PKB phosphorylation status in cells cultured with insulin for 3 days. PKB was only phosphorylated in cells cultured on BM, even though its level remained constant whether the cells were on collagen I or BM substrata. These results are an indirect confirmation that PI 3-kinase activity depends on coordinate signals from insulin and BM. Most importantly, they demonstrate that the PKB pathway, already shown to be a critical determinant of survival in other cell systems, relies on converging insulin and ECM signals in mammary epithelia.
Figure 6.
Phosphorylation of PKB is coordinately regulated by insulin and BM. Mammary cells cultured on either collagen I or BM were stimulated for 15 min with insulin or were left unstimulated. Wortmannin was included in some cultures at the time of insulin addition to show that PKB phosphorylation depended on enzymes within the PI 3-kinase signaling pathway. Some cultures were treated with insulin continuously for 3 d before harvesting. (A) Cell lysates were analyzed by immunoblotting with an antibody specific for phospho-PKB (p-PKB; top). Equivalent amounts of PKB were confirmed by reprobing the blots with anti-PKB antibody (PKB; bottom). (B) The signal from the experiment was quantitated by scanning densitometry and the level of phosphorylation in each sample was normalized to the level of PKB. Note that maximal phosphorylation depended on both insulin treatment and the cells being in contact with BM, and that it was inhibited by wortmannin.
Thus, the ECM dependence of insulin signaling correlates with the ability of BM, but not collagen I, to deliver survival cues.
Pharmacological Inhibitors of Enzymes in the PI 3-Kinase Signaling Pathway Induce Apoptosis of Mammary Epithelial Cells
The experiments described above suggested that the mechanism through which cell–ECM interactions regulate apoptotic fate in mammary epithelial cells is through a control on insulin-mediated PI 3-kinase signaling. To determine whether the PI 3-kinase pathway, or related pathways, were required for survival, the effects of the pharmacological inhibitors LY 294002 and wortmannin were examined in two independent assays for mammary apoptosis.
Mammary cells were cultured on a BM matrix with 10– 100 nM IGF-I, with or without 0.1 or 1 μM LY 294002. In the absence of soluble factors the cells underwent apoptosis (Fig. 7 A, lane 2), and this was suppressed by IGF-I (Fig. 7 A, lanes 3–6). However, IGF-I failed to suppress apoptosis when LY 294002 was included in the cultures (Fig. 7 A, lanes 7–10). We observed low levels of apoptosis at 0.1 μM and very extensive DNA fragmentation at 1 μM LY 294002, a concentration previously shown to inhibit IGF-I signaling and PI 3-kinase activity (Vlahos et al., 1994; Parrizas et al., 1997). Similar results were obtained with wortmannin (data not shown).
Figure 7.
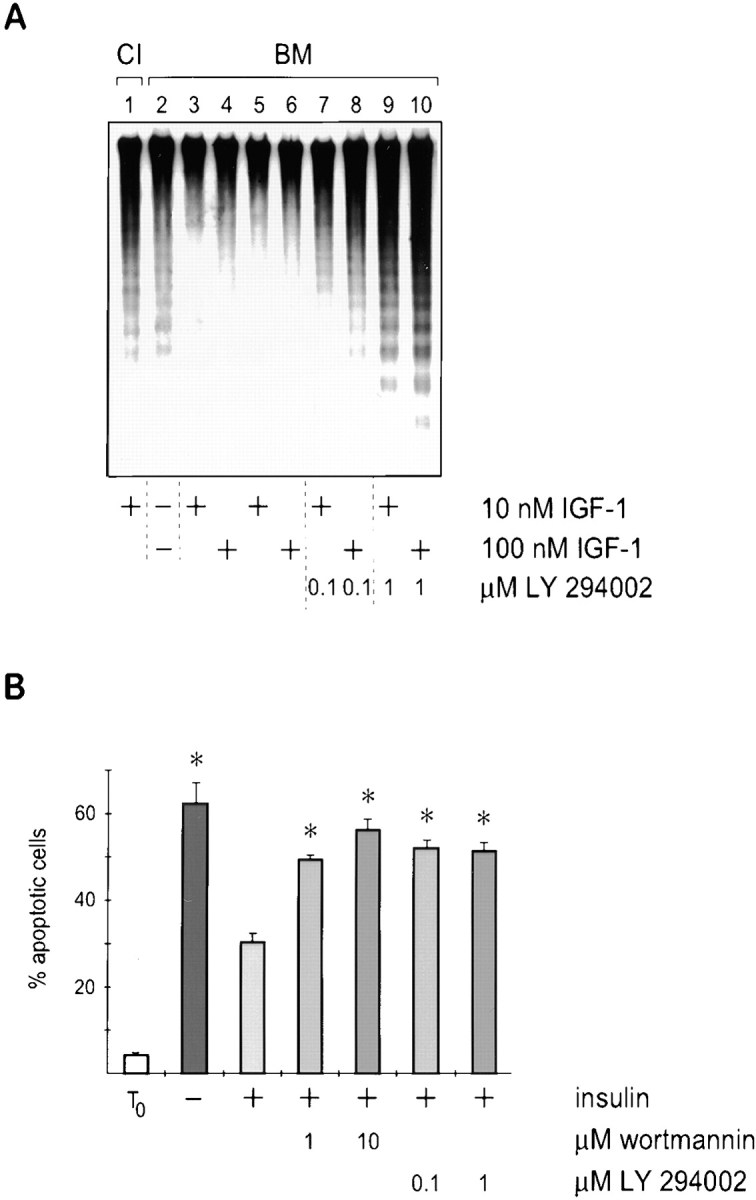
Enzymes in the PI 3-kinase signaling pathway are required to suppress apoptosis of mammary epithelial cells. (A) Southern analysis of DNA isolated from cells cultured on collagen I, or on BM in the absence of factors or with IGF-I and the kinase inhibitor LY 294002. Note the inability of IGF-1 to suppress apoptosis in the presence of PI 3-kinase inhibitor. Vehicle alone (0.1% DMSO) did not itself induce DNA fragmentation (lanes 5 and 6). (B) Quantitative analysis of apoptosis in single cells embedded within BM in the presence of kinase inhibitors. The percentage of apoptotic cells, as determined by nuclear morphology after Hoechst staining, is shown. Results are the mean ± SE of counts from four to six independent cultures from two separate experiments. The values with kinase inhibitors are significantly different to those without (*P < 0.001). Note that insulin failed to suppress apoptosis in mammary cells cultured with 1 μM wortmannin or 0.1 μM LY 294002.
The effect of the kinase inhibitors on suppression of apoptosis by insulin was also examined in the single cell assay. As before, mammary cells cultured within BM matrix were not able to survive in the absence of insulin. Insulin rescued the cells from apoptosis, but low levels of wortmannin (1 μM) and LY 294002 (0.1 μM) blocked survival signaling (Fig. 7 B). The target specificity for wortmannin is fairly broad but includes PI 3-kinase whereas LY 294002 is specific for PI 3-kinase (Vlahos et al., 1994; Ui et al., 1995). Thus, these two experiments indicate that the PI 3-kinase class of enzymes are necessary for mammary cell survival.
Together our results suggest that mammary epithelial cell survival depends upon a functional PI 3-kinase signaling pathway which is normally triggered by insulin when the cells are cultured with a BM substratum. Restricting the activity of this pathway by culture on collagen I results in cell death.
Discussion
The mechanisms that regulate death and survival decisions in epithelial cells are not understood. Yet these are fundamental cellular processes and are essential not only for deciphering the control of normal development, but also for designing strategies to tackle progressive diseases such as breast cancer.
In this study, we have addressed the mechanism whereby BM suppresses apoptosis in primary cultures of mammary epithelial cells and found that this type of ECM controls the ability of insulin to deliver survival signals. We have demonstrated that insulin is necessary to prevent death of mammary cells through the PI 3-kinase pathway, and furthermore that insulin-mediated interaction of PI 3-kinase with IRS-1 and phosphorylation of PKB, a key enzyme downstream in the PI 3-kinase pathway, is influenced by cell interactions with BM. This provides new evidence for the importance of ECM in the growth factor–mediated regulation of cell phenotype. Since α6 and β1 integrins are necessary for mammary cell survival, our work supports the notion that one function of integrins is to regulate growth factor signaling.
Two Signals Are Required for Survival of Mammary Epithelial Cells
Our previous studies, and those of others, have demonstrated that mammary epithelial cells depend on BM for survival (Boudreau et al., 1995; Pullan et al., 1996). We have now found that they require a further signal from soluble ligands to remain alive. Of three hormones tested, insulin, but not hydrocortisone or prolactin, was found to be necessary for suppression of apoptosis (Fig. 1). This survival effect of insulin could be mimicked by IGFs.
Insulin and IGFs have been shown to rescue apoptosis in several cell lines following interleukin-3 or serum withdrawal (Rodriguez-Tarduchy et al., 1992; Harrington et al., 1994; Parrizas et al., 1997). However, although IGFs have been proposed to act as survival factors in mammary gland, this has not been demonstrated directly. For example, overexpression of des(1-3)hIGF-I or IGF-I in mammary glands of transgenic mice delayed involution and the glands showed lower levels of apoptosis, but the target cells were not identified (Hadsell et al., 1996; Neuenschwander et al., 1996). In the experiments performed in our study, purified populations of mouse mammary epithelial cells survived in the presence of insulin or IGFs, indicating that the factors suppress apoptosis by a direct action on the epithelial cells themselves.
Glucocorticoids have also been demonstrated to rescue cells from apoptosis in the mammary gland, although it was not determined whether the hormone acts directly on epithelial cells or through an indirect mechanism (Feng et al., 1995; Lund et al., 1996). In purified mammary epithelial cells hydrocortisone had a minimal effect on survival, and therefore its function in vivo would appear indirect. Thus, although in vivo studies have indicated a role for both IGFs and glucocorticoids in suppressing apoptosis in mammary gland, our study with isolated cells shows that only IGFs act directly on the epithelial cells themselves. IGFs are normally only synthesized by stromal cells in the mammary gland, but their expression is regulated by a synergy between growth hormone and estradiol (Yee et al., 1989; Ruan et al., 1995). Therefore, one explanation for the inhibition of apoptosis by hydrocortisone in vivo is that this steroid can act on stromal cells to alter IGF expression. Alternatively, since IGFBP-5 has been suggested to induce mammary involution, glucocorticoids may inhibit apoptosis in vivo by decreasing IGFBP-5 synthesis, as occurs in cultured fibroblasts (Conover et al., 1995; Tonner et al., 1997).
The ability of prolactin to suppress apoptosis directly in purified mammary epithelial cells was also tested. In agreement with previous studies which showed that prolactin could not rescue mammary apoptosis in vivo, we demonstrated that it also had a negligible effect on epithelial cell survival in culture (Feng et al., 1995; Marti et al., 1997). Prolactin regulates intracellular signaling through a Jak/Stat pathway, which is required for mammary differentiation. Although other cytokines have been proposed to suppress apoptosis through Stat transcription factors, it appears that this pathway is not directly involved in mammary cell survival (Fujio et al., 1997).
Together our data show that, of the hormones tested, insulin is sufficient for the survival of mammary cells cultured on BM. Since prolactin and hydrocortisone, in addition to insulin, are necessary for lactation, the results indicate that mammary cell survival does not depend on differentiation. Moreover, as cells on collagen I undergo apoptosis even in the presence of all three hormones, the survival mechanism would appear to have more to do with cell–ECM interactions than with differentiation.
Laminin Is a Survival Ligand for Mammary Cells
Cell–ECM interactions are known to suppress apoptosis in epithelial cells (Meredith et al., 1993; Frisch and Francis, 1994; Khwaja et al., 1997). However, such studies are based on comparing the survival potential of suspension-cultured epithelial cell lines with those adhering to ECM in short term assays. We have developed a model where primary mammary cells isolated directly from tissue are maintained on physiological substrata in longer term assays. These cells have not been selected for their ability to form cell lines and therefore retain an apoptotic potential that is as close as possible to that in vivo. They undergo apoptosis specifically after culture for several days on tissue culture plastic or collagen I, but not on BM (Pullan et al., 1996). The cells plated on a BM substratum form multicellular structures resembling alveoli in vivo (Barcellos-Hoff et al., 1989; Aggeler et al., 1991). However, this three-dimensional architecture did not appear to play a role in suppression of apoptosis (Fig. 2). Under conditions where the cells remained as monolayers, apoptosis was efficiently suppressed by diluted BM proteins precipitating over the cells.
Further verification that BM had a direct survival signaling role came from studies using function-blocking antibodies. Using a single cell assay developed previously for analysis of differentiation (Streuli et al., 1991), we found that although insulin was able to significantly suppress apoptosis in cells cultured within a three-dimensional BM gel, it was not able to do so if anti-laminin antibodies were included (Fig. 3). Laminin has previously been shown to act as a survival ligand for other cell lineages including fibrosarcoma and neuroblastoma cells and has been implicated in preventing apoptosis during myogenesis (Kim et al., 1994; Vachon et al., 1996; Bozzo et al., 1997; Vachon et al., 1997). Our results extend these previous findings and show that laminin also has a survival function in mammary epithelium. However, laminin may not be a survival ligand for all cells, since endothelial cells underwent apoptosis on laminin substrata but not on fibronectin or vitronectin (Wary et al., 1996). Thus, distinct cell types have a different requirement for ECM to rescue them from apoptosis. It remains to be determined whether or not the survival response to different ECM ligands is mediated through similar signaling pathways.
Integrins Are Survival Receptors in Mammary Cells
In several cell systems, integrins have been shown to be required for mediating a survival response (Frisch and Ruoslahti, 1997). We tested the possibility that integrins delivered survival signals in mammary cells by examining the survival of single cells in BM gels after treatment with function-blocking antibodies, and found that both α6 and β1 integrins were necessary to prevent the cells from undergoing apoptosis. Although β1 integrins have been shown to be required for mammary epithelial cell survival, our results augment these studies by providing the first direct evidence that α6 integrin functions in such a pathway (Boudreau et al., 1995; Howlett et al., 1995; Fig. 4). These findings have recently been confirmed in our experiments with primary human breast epithelia, where anti–α6 and anti–β1 integrin antibodies induced cell death, although antibodies to the α2 subunit did not. The latter result indicates a specificity of response as mammary cells express significant levels of α2 integrin, but it also suggests that the α2β1 integrin, a receptor for collagen, is not involved with suppressing mammary apoptosis.
The β1 and β3 integrin subunits have previously been shown to be survival receptors (Brooks et al., 1994; Boudreau et al., 1995; Howlett et al., 1995; Zhang et al., 1995; Scatena et al., 1998). RGD is a peptide substrate for αvβ3 integrin but we found that it had no effects on mammary cell survival. Furthermore, in preliminary immunostaining experiments with anti–αv integrin antibodies, we have only detected this subunit in the epithelial cells of virgin mammary gland and it appeared to be completely absent from pregnant and lactating tissue. Therefore, our data would not support the possibility that it is a survival receptor for mammary cells isolated from pregnant mammary gland, even though it does have this role in endothelia (Brooks et al., 1994; Scatena et al., 1998). In addition to the β1 integrin subunit, mammary cells also express β4 integrin, and both of these can partner the α6 subunit. Therefore, our data are not yet sufficient to confirm that laminin suppresses apoptosis through a direct cell interaction with the α6β1 integrin heterodimer, neither do they exclude the possibility that other integrin subunits are involved in survival. However, they do demonstrate that laminin is a ligand for survival and that the α6 and β1 subunits can act as survival receptors.
The metastatic breast cancer cell line, MDA-MB-435 expresses α6β1 integrin. Tumors induced by a derivative cell line in which α6β1 was functionally ablated were much smaller those of the parental cells, and showed a sixfold higher apoptotic/mitotic index (Wewer et al., 1997). These results suggest that α6 integrin provides an anti-apoptotic signal in vivo as well as in culture and together with the present study, they may explain why higher levels of α6 integrin in human breast carcinomas correlate with an increased likelihood of patient morbidity (Friedrichs et al., 1995). Moreover, they suggest that α6 integrin–ligand interactions might represent a target for therapeutic intervention in breast disease, especially in combination with agents which inhibit growth factor–mediated survival signals.
Receptor Cross Talk and the Control of Apoptosis
Since both BM and insulin were required for sustained prevention of apoptosis in mammary cells, it was possible that these ligands triggered either parallel or convergent survival pathways. An abundance of studies indicate that PI 3-kinase is essential for suppressing apoptosis in other cell systems, possibly through PKB which has been implicated as a downstream regulator of survival (Yao and Cooper, 1995; Minshall et al., 1996; Dudek et al., 1997; Kauffmann-Zeh et al., 1997; Kennedy et al., 1997; Khwaja et al., 1997; Kulik et al., 1997; Parrizas et al., 1997; Crowder and Freeman, 1998). Our experiments using inhibitors also demonstrated a requirement for PI 3-kinase in mammary cell survival (Fig. 7). Therefore, we examined whether this pathway was independently regulated by the two separate ligands or if BM controlled the ability of insulin to trigger its phosphorylation cascade, by measuring the levels of phosphotyrosine in its proximal signaling proteins.
Insulin rapidly induced tyrosine phosphorylation of its receptor. However, the signaling events downstream of the insulin receptor resulting in IRS-1 tyrosine phosphorylation and its association with PI 3-kinase were only propagated in cells cultured on BM and not on collagen I (Fig. 5). These results contrast with a study using CHO cells overexpressing insulin receptor, which showed that transient adhesion to fibronectin >20 min enhanced the insulin-induced phosphorylation of both the receptor and IRS-1 (Guilherme et al., 1998). Our data support the conclusion that ECM amplifies insulin signaling, but extends it by showing that once nontransfected primary epithelial cells have become established within an ECM environment over several days, they develop a selective sensitivity to growth factor signaling. Thus, the cellular environment determines whether the insulin signal can be propagated efficiently, and this only occurs when the cells contact a BM but not when they are on collagen I. Indeed, the results indicate the existence of an ECM-dependent restriction point in insulin signaling, which occurs downstream of insulin receptor phosphorylation.
The conclusion that BM and insulin cooperate to drive insulin signaling was confirmed in further experiments where we examined PKB phosphorylation, using both short and long term treatments with insulin (Fig. 6). Cell– matrix interactions had a similar effect of enhancing EGF signaling in MDCK cells, although in that study the PKB activation was examined in cells briefly attached to an ECM in comparison with those detached from a substratum (Khwaja et al., 1997). Together, these complementary results indicate that both the short and long term activation of enzymes in the PI 3-kinase signaling pathway requires adherent cells to be in the correct ECM microenvironment, which contributes to both EGF and insulin signaling.
At the current time we do not know whether PKB is absolutely required for mammary cell survival, but given the results from other cell systems, this seems likely. Candidates for the downstream link between PKB and the apoptotic machinery include glycogen synthase kinase-3, procaspase-9, and the pro-apoptotic protein Bad (Datta et al., 1997; del Peso et al., 1997; Cardone et al., 1998; Pap and Cooper, 1998). Although the phosphorylation of PKB and Bad have been dissociated from each other, primary cultures of mammary epithelial cells do express Bad, which therefore remains a potential target for regulation by PKB (Scheid and Duronio, 1998; our unpublished data).
Adhesion to ECM has previously been shown to suppress apoptosis in several cell types (Meredith et al., 1993; Frisch and Francis, 1994; Kim et al., 1994; Vachon et al., 1996; Wary et al., 1996; Bozzo et al., 1997; Khwaja et al., 1997). In addition, cell–ECM interactions trigger integrin-mediated downstream phosphorylation cascades involving MAP kinase and PI 3-kinase, providing possible mechanisms for ECM control of survival (Khwaja et al., 1997; King et al., 1997; Lin et al., 1997). However, in the mammary gland model we have now demonstrated that although integrin signaling is necessary for survival, sustained cell interactions with a BM substratum alone are not sufficient to suppress apoptosis. In addition, insulin or IGFs are also required. Similar conclusions have been reached in studies of the cell cycle where serum factors and ECM are both required for MAP kinase activation, cyclin E–cdk2 activation, and retinoblastoma phosphorylation (Zhu et al., 1996; Renshaw et al., 1997). Dual signals from soluble factors and ECM are also implicated in the control of mammary differentiation, where prolactin and BM are both necessary for triggering the prolactin signaling cassette, Stat5 DNA binding, and milk protein gene transcription (Streuli et al., 1995; Edwards et al., 1998).
Although our work shows that both laminin and insulin act as survival ligands for mammary cells, we cannot rule out the possibility that other types of ligand are important in regulating survival. Insulin, for example, was only able to suppress apoptosis of single mammary cells cultured within BM by about twofold, suggesting that other factors may play a role in mammary cell survival in vivo. Preliminary studies with primary cultures of human mammary epithelial cells, have demonstrated that apoptosis in the single cell assay can be reduced by insulin, but is further suppressed by EGF. But with mouse mammary cells, EGF does not have an additional survival effect over and above insulin (Oliver, J., and C.H. Streuli, unpublished data). Therefore, it may be that additional cytokines can suppress apoptosis still further than insulin and BM in the mouse system. Even if other mammary survival factors remain to be identified, our data are still sufficient to demonstrate that both insulin and integrin contribute to the survival of mammary cells.
In summary, these studies indicate that an important function of cell–ECM interactions is to modulate growth factor and cytokine responses. This suggests that current thinking about growth factor signaling in adherent cells should include a component from the ECM. One mechanism might be via direct or indirect associations between integrin and growth factor receptors, as has been demonstrated for αvβ3 integrin and the insulin and PDGF receptors, or with integrin and downstream components in the insulin signaling pathway, as shown with αvβ3 integrin and IRS-1 (Vuori and Ruoslahti, 1994; Schneller et al., 1997). The role of integrins and the cytoskeleton in signal transduction may be to provide a scaffold whereby components of growth factors cassettes are assembled into discreet domains within the cell, so that they can propagate signals efficiently. Indeed, a modular nature for membrane signaling complexes has been proposed, which may explain the recruitment of signaling proteins to cytoskeletal structures such as adhesion plaques (Miyamoto et al., 1995; Plopper et al., 1995; Pawson and Scott, 1997; Simons and Ikonen, 1997). Our current goal is to address this issue by determining whether the macromolecular organization of insulin signaling proteins and integrins in mammary cells cultured on BM are different to those in cells on collagen I.
Acknowledgments
The authors are grateful in particular to Dr. Andrew Gilmore and also to Drs. Neil Anderson, Gwynneth Edwards, David Garrod, Teresa Klinowska, and John Hickman for critical review of the manuscript.
Abbreviations used in this paper
- BM
basement membrane
- ECM
extracellular matrix
- EHS
Engelbreth-Holm-Swarm tumor
- IGF
insulin-like growth factor
- IRS
insulin receptor substrate
- PI 3-kinase
phosphatidylinositol 3-kinase
- PKB
protein kinase B
Footnotes
C.H. Streuli is a Wellcome Senior Fellow in Basic Biomedical Science. C. Dive is a Lister Fellow.
References
- Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99:407–417. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ice and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzo C, Bellomo G, Silengo L, Tarone G, Altruda F. Soluble integrin ligands and growth factors independently rescue neuroblastoma cells from apoptosis under nonadherent conditions. Exp Cell Res. 1997;237:326–337. doi: 10.1006/excr.1997.3777. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu TH, Klier G, Cheresh DA. Integrin alpha(v)beta(3) antagonists promote tumor-regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Conover CA, Clarkson JT, Bale LK. Effect of glucocorticoid on insulin-like growth factor (IGF) regulation of IGF-binding protein expression in fibroblasts. Endocrinology. 1995;136:1403–1410. doi: 10.1210/endo.136.4.7534698. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell- intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Edwards, G.M., and C.H. Streuli. 1998. Preparing a polyclonal antibody to mouse β1 integrin with function-blocking activity. In Integrin Protocols. A. Howlett, editor. Humana Press Inc., Totowa, NH. In press.
- Edwards GM, Wilford FH, Liu XW, Hennighausen L, Djiane J, Streuli CH. Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J Biol Chem. 1998;273:9495–9500. doi: 10.1074/jbc.273.16.9495. [DOI] [PubMed] [Google Scholar]
- Feng ZW, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell-death in the mouse mammary gland. J Cell Biol. 1995;131:1095–1103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chanhui PY. Control of adhesion-dependent cell-survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Kunisada K, Hirota H, Yamuchi-Takihara K, Kishimoto T. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest. 1997;99:2898–2905. doi: 10.1172/JCI119484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, Torres K, Czech MP. Cross-talk between insulin receptor and integrin alpha5 beta1 signaling pathways. J Biol Chem. 1998;273:22899–22903. doi: 10.1074/jbc.273.36.22899. [DOI] [PubMed] [Google Scholar]
- Hadsell DL, Greenberg NM, Fligger JM, Baumrucker CR, Rosen JM. Targeted expression of des(1-3) human insulin-like growth-factor-I in transgenic mice influences mammary-gland development and Igf-binding protein expression. Endocrinology. 1996;137:321–330. doi: 10.1210/endo.137.1.8536631. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bennett MR, Fanidi A, Evan GI. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO (Eur Mol Biol Organ) J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta-1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez P, Viciana, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez P, Viciana, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO (Eur Mol Biol Organ) J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Schnaper HW, Nomizu M, Yamada Y, Kleinman HK. Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res. 1994;54:5005–5010. [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Aplin AE, Shen Y, Chen QM, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Chen QM, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between Ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dano K, Werb Z. Two distinct phases of apoptosis in mammary-gland involution—proteinase-independent and proteinase-dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti A, Feng Z, Altermatt HJ, Jaggi R. Milk accumulation triggers apoptosis of mammary epithelial cells. Eur J Cell Biol. 1997;73:158–165. [PubMed] [Google Scholar]
- Meredith JE, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A, Streuli C. Epithelial apoptosis. Bioessays. 1997;19:711–720. doi: 10.1002/bies.950190812. [DOI] [PubMed] [Google Scholar]
- Miller TM, Tansey MG, Johnson EM, Jr, Creedon DJ. Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization- and insulin-like growth factor I-mediated survival of cerebellar granule cells. J Biol Chem. 1997;272:9847–9853. doi: 10.1074/jbc.272.15.9847. [DOI] [PubMed] [Google Scholar]
- Minshall C, Arkins S, Freund GG, Kelley KW. Requirement for phosphatidylinositol 3′-kinase to protect hemopoietic progenitors against apoptosis depends upon the extracellular survival factor. J Immunol. 1996;156:939–947. [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function—molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Grammer TC, Wang LM, Sun XJ, Pierce JH, Blenis J, White MF. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- Neuenschwander S, Schwartz A, Wood TL, Roberts CT, Henninghausen L, Leroith D. Involution of the lactating mammary gland is inhibited by the Igf system in a transgenic mouse model. J Clin Invest. 1996;97:2225–2232. doi: 10.1172/JCI118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth-factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan, S., and C.H. Streuli. 1996. The mammary gland epithelial cell. In Epithelial Cell Culture. A. Harris, editor. Cambridge University Press. 97–121.
- Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO (Eur Mol Biol Organ) J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G, Collins MK, Garcia I, Lopez-Rivas A. Insulin-like growth factor-I inhibits apoptosis in IL-3-dependent hemopoietic cells. J Immunol. 1992;149:535–540. [PubMed] [Google Scholar]
- Ruan WF, Catanese V, Wieczorek R, Feldman M, Kleinberg DL. Estradiol enhances the stimulatory effect of insulin-like growth factor-I (Igf-I) on mammary development and growth hormone-induced Igf-I messenger ribonucleic acid. Endocrinology. 1995;136:1296–1302. doi: 10.1210/endo.136.3.7867584. [DOI] [PubMed] [Google Scholar]
- Runswick SK, Garrod DR, Streuli CH. The differential expression of desmocollin isoforms in mammary epithelia. Biochem Soc Trans. 1996;24:S346. doi: 10.1042/bst024346s. [DOI] [PubMed] [Google Scholar]
- Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. Alpha v beta 3 integrin associates with activated insulin and PDGF beta receptors and potentiates the biological activity of PDGF. EMBO (Eur Mol Biol Organ) J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H. 1995. Basement membrane in the control of mammary gland function. In Intercellular Signalling in the Mammary Gland. C.J. Wilde, M. Peaker, and C.H. Knight, editors. Plenum Press, New York. 141–151.
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation—basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CBA, Burdon TG, Schindler C, Watson CJ. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XJ, Miralpeix M, Myers MG, Jr, Glasheen EM, Backer JM, Kahn CR, White MF. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub M, Wang Y, Szczesny TM, Kleinman HK. Epidermal growth factor or transforming growth factor-alpha is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc Natl Acad Sci USA. 1990;87:4002–4006. doi: 10.1073/pnas.87.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonner E, Barber MC, Travers MT, Logan A, Flint DJ. Hormonal control of insulin-like growth factor binding protein-5 production in the involuting mammary gland of the rat. Endocrinology. 1997;138:5101–5107. doi: 10.1210/endo.138.12.5619. [DOI] [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH, Xu H, Liu L, Loechel F, Hayashi Y, Arahata K, Reed JC, Wewer UM, Engvall E. Integrins (α7β1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adapter protein Shc couples a class of integrins to the control of cell-cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol. 1997;151:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N. Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol. 1989;3:509–517. doi: 10.1210/mend-3-3-509. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Vuori K, Reed JC, Ruoslahti E. The alpha-5-beta-1 integrin supports survival of cells on fibronectin and up-regulates bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell-cycle progression linked to the expression of cyclin D1, activation of cyclin E-Cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]