Abstract
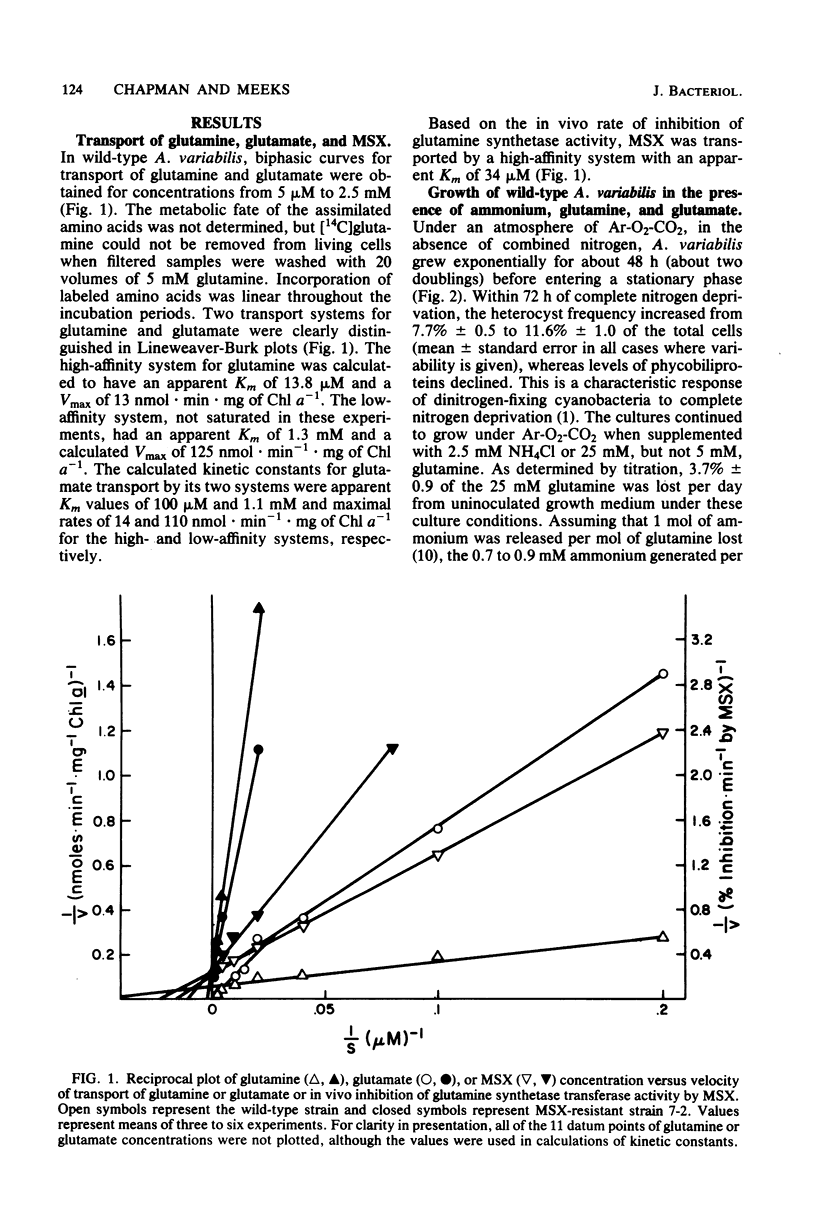
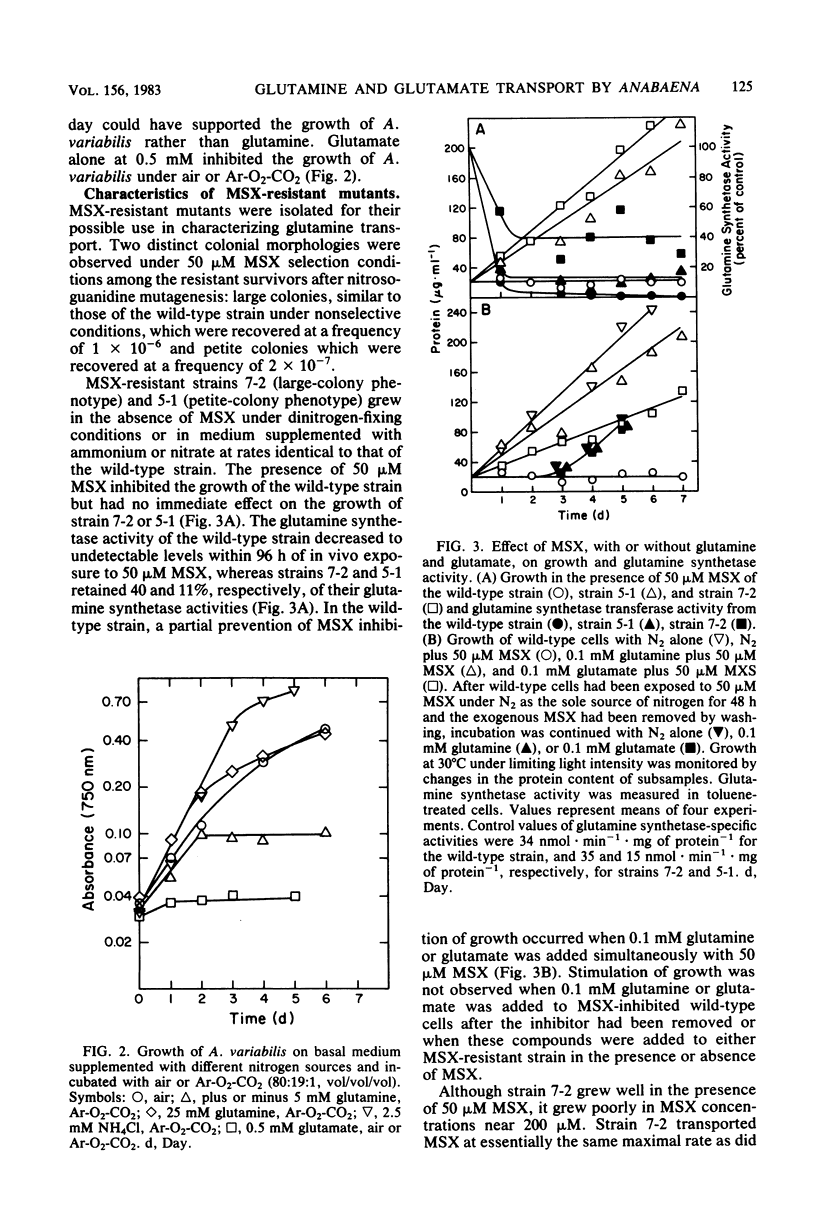
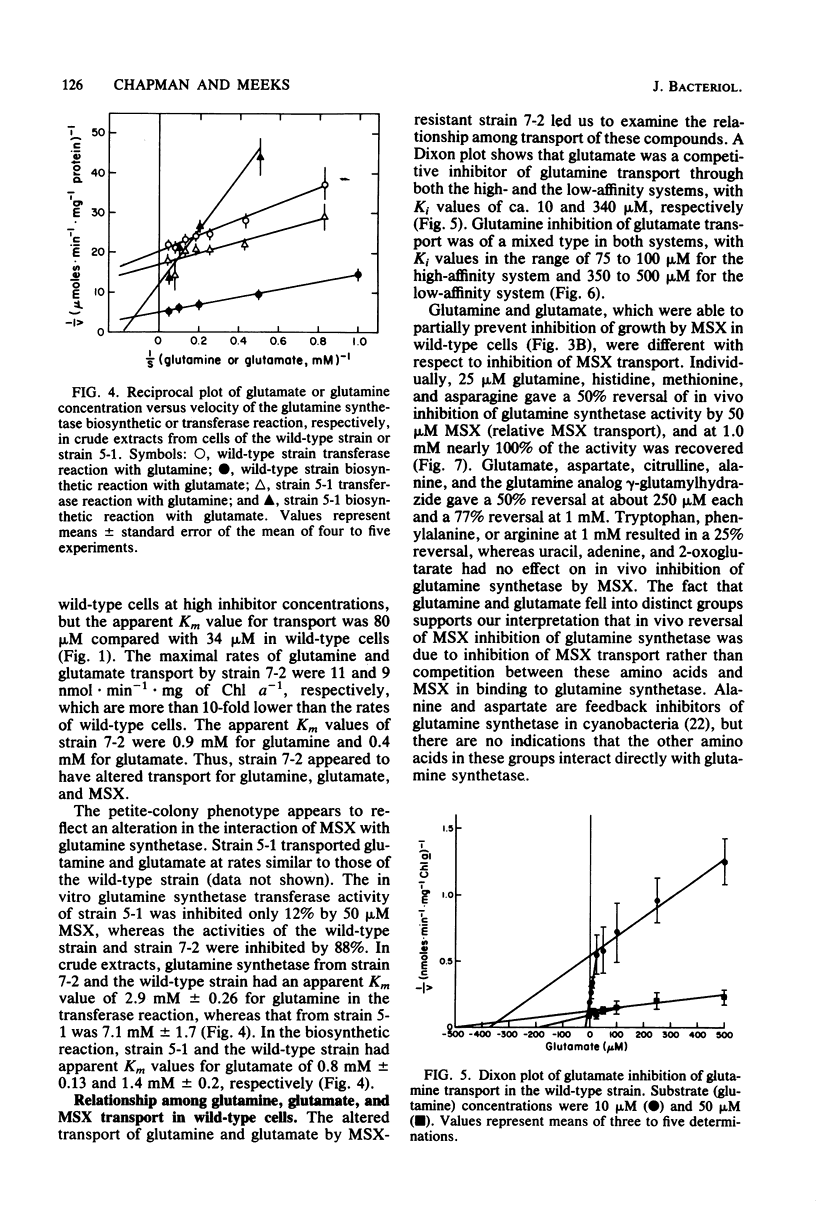
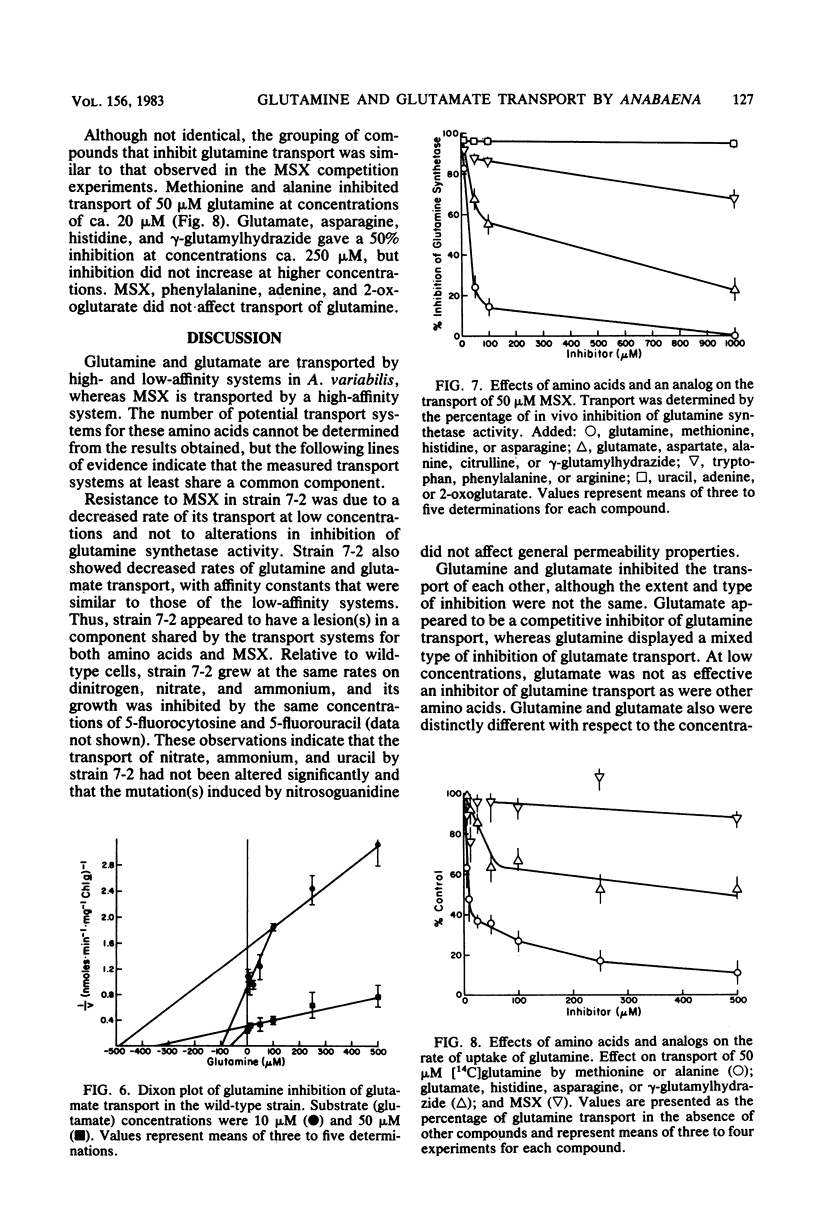
Anabaena variabilis, a dinitrogen-fixing cyanobacterium, has high- and low-affinity systems for the transport of glutamine and glutamate. The high-affinity systems have Km values of 13.8 and 100 microM and maximal rates of 13.2 and 14.4 nmol X min-1 X mg of chlorophyll a-1 for glutamine and glutamate, respectively. The low-affinity systems have Km values of 1.1 and 1.4 mM and maximal rates of 125 and 100 nmol X min-1 X mg of chlorophyll a-1 for glutamine and glutamate, respectively. Glutamine was unable to support growth of A. variabilis in the absence of any other nitrogen source, and glutamate alone at 500 microM was inhibitory to its growth. The analog L-methionine-DL-sulfoximine (MSX) was transported by a high-affinity system with a Km of 34 microM. Competition experiments and the transport characteristics of a specific class of MSX-resistant mutants imply that glutamine, glutamate, and MSX share a common component for transport. A second class of MSX-resistant mutants had a glutamine synthetase activity with altered affinity constants for glutamine and glutamate relative to the wild-type enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Adams D. G., Carr N. G. The developmental biology of heterocyst and akinete formation in cyanobacteria. Crit Rev Microbiol. 1981;9(1):45–100. doi: 10.3109/10408418109104486. [DOI] [PubMed] [Google Scholar]
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Haury J. F., Wolk C. P. Isolation and preliminary characterization of auxotrophs of a filamentous Cyanobacterium. J Bacteriol. 1977 Mar;129(3):1556–1562. doi: 10.1128/jb.129.3.1556-1562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Cooper A. J., Meister A. On the utilization of L-glutamine by glutamate dehydrogenase. Biochem Biophys Res Commun. 1976 May 17;70(2):373–380. doi: 10.1016/0006-291x(76)91056-1. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The hisP protein, a known histidine transport component in Salmonella typhimurium, is also an arginine transport component. J Bacteriol. 1973 Oct;116(1):107–113. doi: 10.1128/jb.116.1.107-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee-Kaden J., Simonis W. Amino acid uptake and energy coupling dependent on photosynthesis in Anacystis nidulans. J Bacteriol. 1982 Jul;151(1):229–236. doi: 10.1128/jb.151.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Wycoff K. L., Chapman J. S., Enderlin C. S. Regulation of expression of nitrate and dinitrogen assimilation by anabaena species. Appl Environ Microbiol. 1983 Apr;45(4):1351–1359. doi: 10.1128/aem.45.4.1351-1359.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Premakumar R., Sorger G. J., Gooden D. Repression of nitrate reductase in Neurospora studied by using L-methionine-DL-sulfoximine and glutamine auxotroph gln-1b. J Bacteriol. 1980 Jul;143(1):411–415. doi: 10.1128/jb.143.1.411-415.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Steimer-Veale K., Brenchley J. E. Characterization of Salmonella typhimurium strains sensitive and resistant to methionine sulfoximine. J Bacteriol. 1974 Sep;119(3):848–856. doi: 10.1128/jb.119.3.848-856.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Meeks J. C., Wolk C. P., Shaffer P. W., Austin S. M. Formation of glutamine from [13n]ammonia, [13n]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J Bacteriol. 1977 Mar;129(3):1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Physiology and cytological chemistry blue-green algae. Bacteriol Rev. 1973 Mar;37(1):32–101. doi: 10.1128/br.37.1.32-101.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


