Abstract
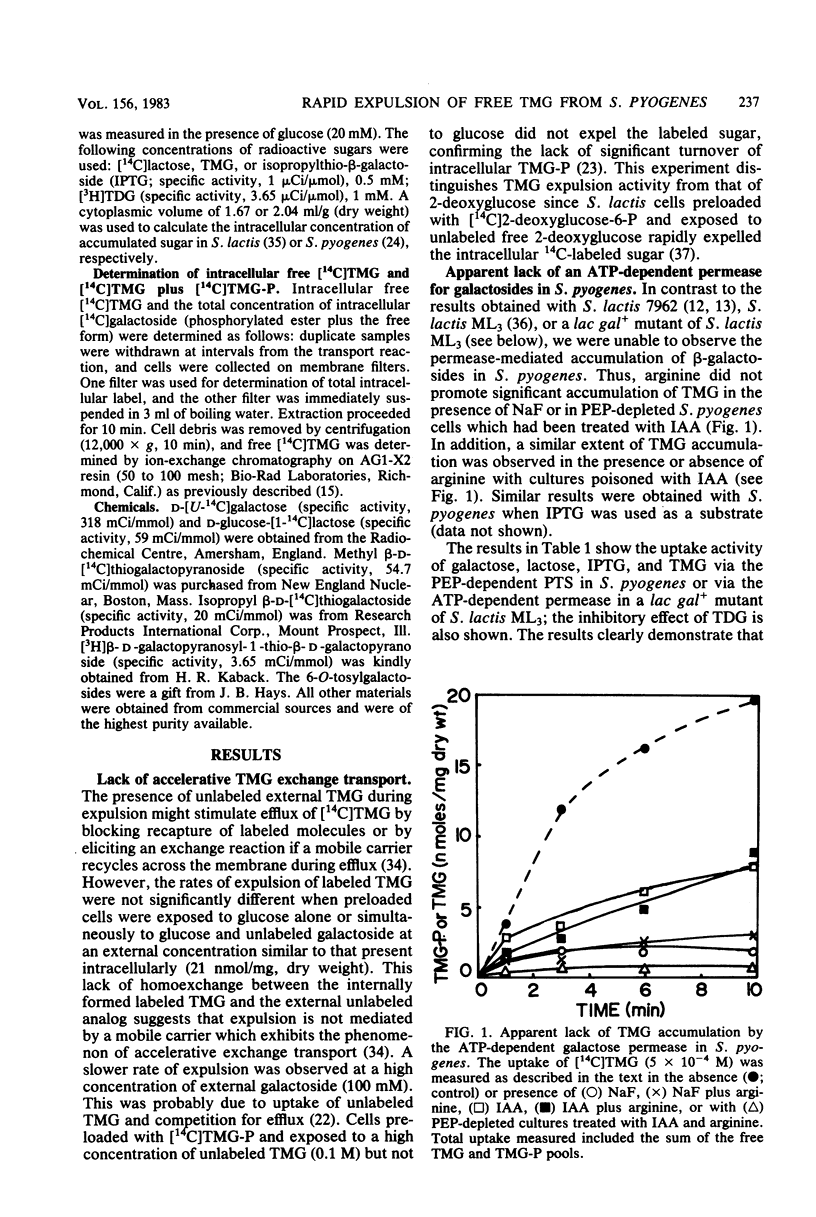
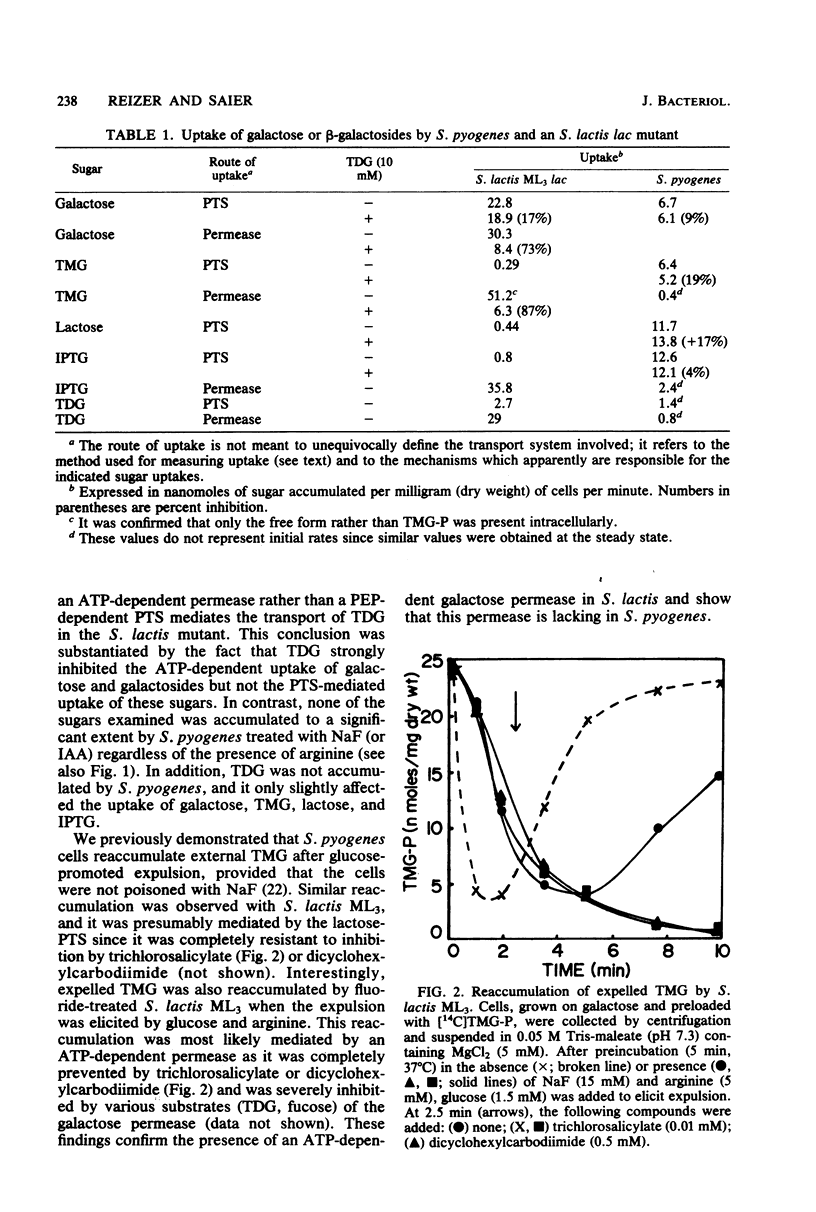
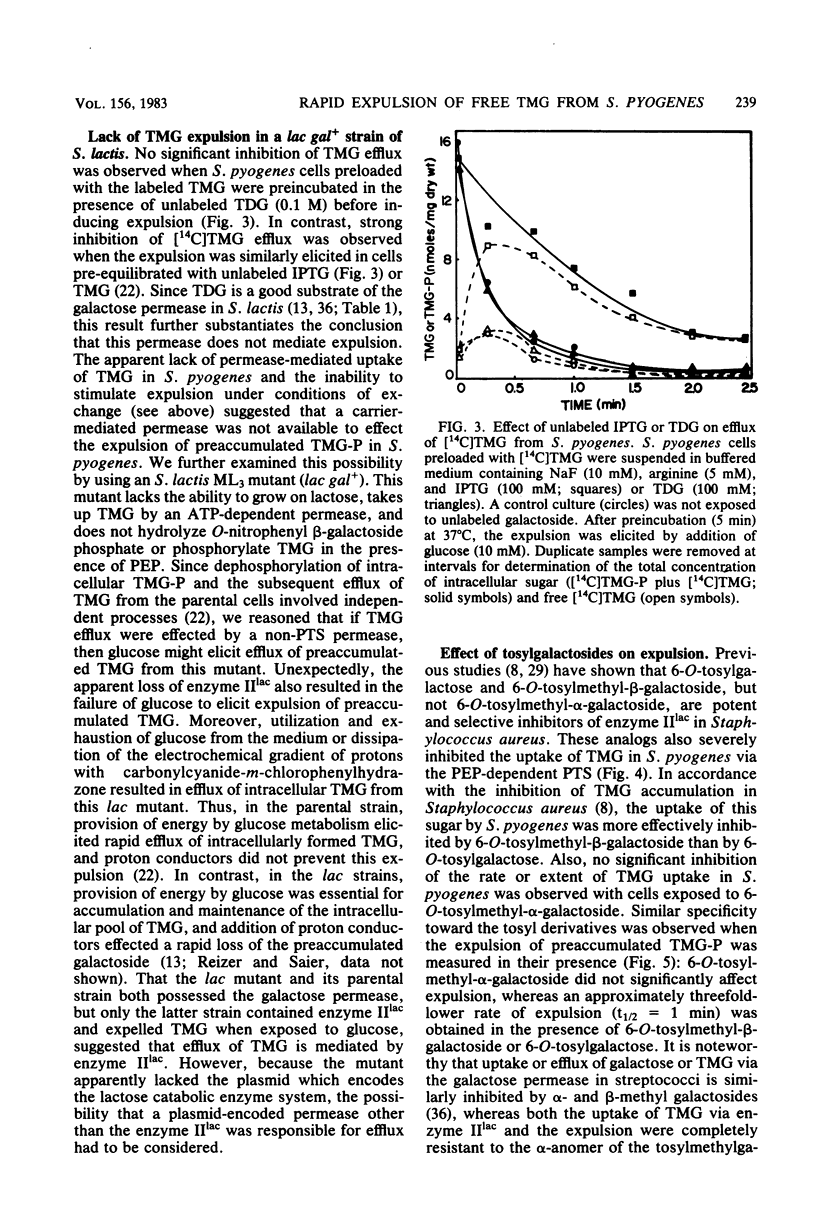
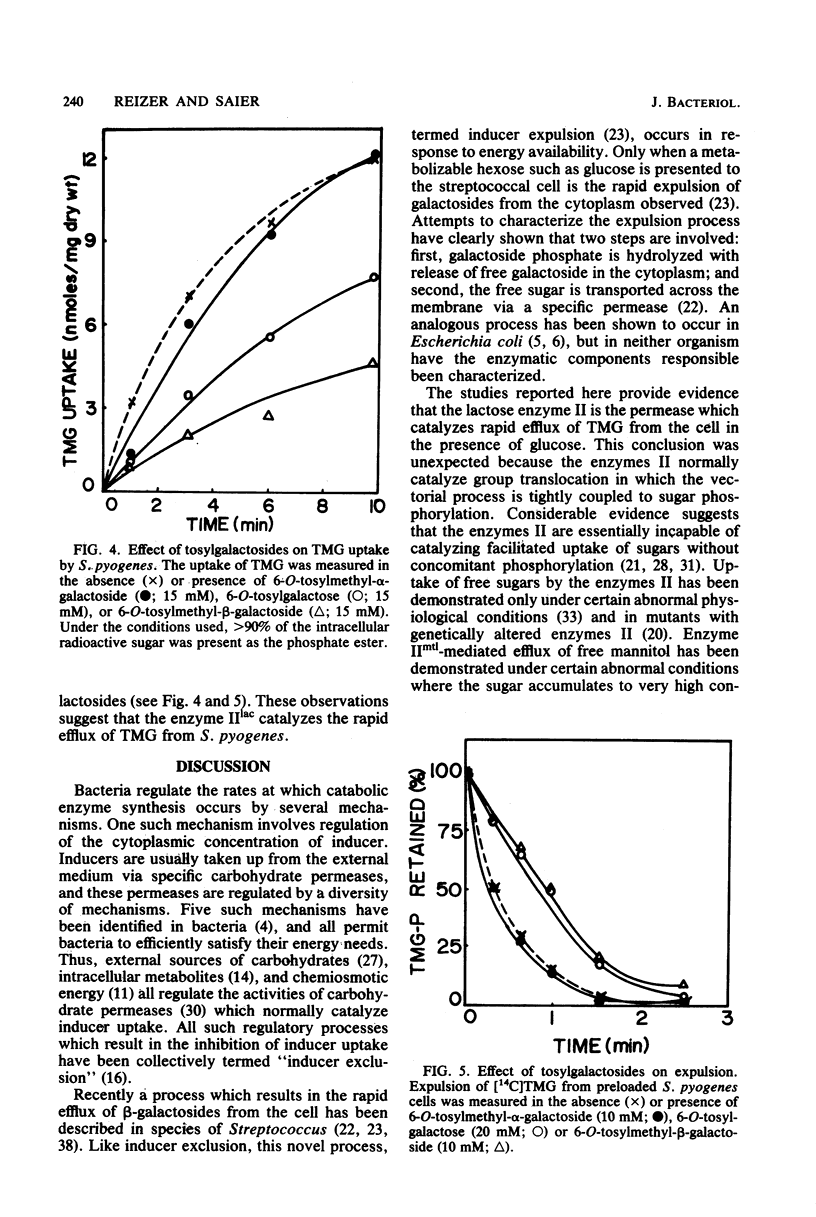
Streptococcus pyogenes accumulated thiomethyl-beta-galactoside as the 6-phosphate ester due to the action of the phosphoenolpyruvate:lactose phosphotransferase system. Subsequent addition of glucose resulted in rapid efflux of the free galactoside after intracellular dephosphorylation (inducer expulsion). Efflux was shown to occur in the apparent absence of the galactose permease, but was inhibited by substrate analogs of the lactose enzyme II and could not be demonstrated in a mutant of S. lactis ML3 which lacked this enzyme. The results suggest that the enzymes II of the phosphotransferase system can catalyze the rapid efflux of free sugar under appropriate physiological conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haguenauer R., Kepes A. NaF inhibition of phosphorylation and dephosphorylation involved in -methyl-D glucoside transport in E. coli K 12. A pH dependant phenomenon sensitive to uncoupling agents. Biochimie. 1972;54(4):505–512. doi: 10.1016/s0300-9084(72)80235-9. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. B., Sussman M. L., Glass T. W. Inhibition by 6-O-tosyl galactosides of beta-galactoside phosphorylation and transport by the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1975 Nov 25;250(22):8834–8839. [PubMed] [Google Scholar]
- Heller K., Röschenthaler R. beta-D-phosphogalactoside galactohydrolase of Streptococcus faecalis and the inhibition of its synthesis by glucose. Can J Microbiol. 1978 May;24(5):512–519. doi: 10.1139/m78-084. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Galactoside accumulation associated with ion movements in Streptococcus lactis. Biochem Biophys Res Commun. 1972 Nov 1;49(3):615–620. doi: 10.1016/0006-291x(72)90455-x. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton T., Hartman P. E., Stratis J. P., Lee T. L., Davis A. T. Chemotaxis of Salmonella typhimurium to amino acids and some sugars. J Bacteriol. 1978 Feb;133(2):708–716. doi: 10.1128/jb.133.2.708-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Bromberg F. G., Roseman S. Characterization of constitutive galactose permease mutants in Salmonella typhimurium. J Bacteriol. 1973 Jan;113(1):512–514. doi: 10.1128/jb.113.1.512-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Catalytic activities associated with the enzymes II of the bacterial phosphotransferase system. J Supramol Struct. 1980;14(3):281–294. doi: 10.1002/jss.400140303. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Young W. S., 3rd, Roseman S. Utilization and transport of hexoses by mutant strains of Salmonella typhimurium lacking enzyme I of the phosphoenolpyruvate-dependent phosphotransferase system. J Biol Chem. 1971 Sep 25;246(18):5838–5840. [PubMed] [Google Scholar]
- Simoni R. D., Smith M. F., Roseman S. Resolution of a staphylococcal phosphotransferase system into four protein components and its relation to sugar transport. Biochem Biophys Res Commun. 1968 Jun 10;31(5):804–811. doi: 10.1016/0006-291x(68)90634-7. [DOI] [PubMed] [Google Scholar]
- Solomon E., Miyal K., Lin E. C. Membrane translocation of mannitol in Escherichia coli without phosphorylation. J Bacteriol. 1973 May;114(2):723–728. doi: 10.1128/jb.114.2.723-728.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Galactose transport systems in Streptococcus lactis. J Bacteriol. 1980 Nov;144(2):683–691. doi: 10.1128/jb.144.2.683-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


