Abstract
α-Syntrophin is a scaffolding adapter protein expressed primarily on the sarcolemma of skeletal muscle. The COOH-terminal half of α-syntrophin binds to dystrophin and related proteins, leaving the PSD-95, discs-large, ZO-1 (PDZ) domain free to recruit other proteins to the dystrophin complex. We investigated the function of the PDZ domain of α-syntrophin in vivo by generating transgenic mouse lines expressing full-length α-syntrophin or a mutated α-syntrophin lacking the PDZ domain (ΔPDZ). The ΔPDZ α-syntrophin displaced endogenous α- and β1-syntrophin from the sarcolemma and resulted in sarcolemma containing little or no syntrophin PDZ domain. As a consequence, neuronal nitric oxide synthase (nNOS) and aquaporin-4 were absent from the sarcolemma. However, the sarcolemmal expression and distribution of muscle sodium channels, which bind the α-syntrophin PDZ domain in vitro, were not altered. Both transgenic mouse lines were bred with an α-syntrophin–null mouse which lacks sarcolemmal nNOS and aquaporin-4. The full-length α-syntrophin, not the ΔPDZ form, reestablished nNOS and aquaporin-4 at the sarcolemma of these mice. Genetic crosses with the mdx mouse showed that neither transgenic syntrophin could associate with the sarcolemma in the absence of dystrophin. Together, these data show that the sarcolemmal localization of nNOS and aquaporin-4 in vivo depends on the presence of a dystrophin-bound α-syntrophin PDZ domain.
Keywords: syntrophin; aquaporin; dystrophin complex; neuronal nitric oxide synthase; PDZ domain
Introduction
The syntrophins are a family of scaffolding proteins that contain multiple protein interaction domains (Froehner et al., 1987). All five syntrophins, α, β1, β2, γ1, and γ2, contain two pleckstrin homology (PH)* domains, a PSD-95, discs-large, ZO-1 (PDZ) domain, and a syntrophin-unique (SU) COOH-terminal region (Ahn et al., 1994; Adams et al., 1995; Piluso et al., 2000). Using these protein interaction domains, syntrophins are capable of binding multiple proteins simultaneously to form functional complexes.
Interest in the syntrophins was originally prompted by the observation that syntrophin was associated with dystrophin and other members of the dystrophin protein family, including utrophin and dystrobrevin (Butler et al., 1992, Ahn and Kunkel, 1995, Dwyer and Froehner, 1995, Yang et al., 1995). Mutations in the dystrophin gene lead to Duchenne and Becker muscular dystrophies (Hoffman et al., 1987). Syntrophins bind to two sites located in the COOH-terminal region of dystrophin such that two syntrophins can attach to one dystrophin (Newey et al., 2000). Dystrophin also binds to dystrobrevin, a protein-containing sequence homology with dystrophin (Sadoulet-Puccio et al., 1996; Peters et al., 1998) and which can also bind two syntrophins (Newey et al., 2000). Thus, the dystrophin complex can bind up to four syntrophins, providing a scaffold for multiple functionally interdependent signaling proteins.
α-Syntrophin is the predominant syntrophin isoform in skeletal and cardiac muscle. In skeletal muscle, α-syntrophin is present on the sarcolemma of all fibers, whereas β1-syntrophin is present on the sarcolemma of only fast type II fibers (Peters et al., 1997). Skeletal muscle β2-syntrophin is found at high levels only at the postsynaptic membrane of the neuromuscular junctions (Peters et al., 1994; Kramarcy and Sealock, 2000). γ2-Syntrophin is also found in skeletal muscle where it has been reported to be located at the sarcolemma (Piluso et al., 2000).
The second PH domain and the highly conserved SU domain are required for interaction with dystrophin (Ahn and Kunkel, 1995; Kachinsky et al., 1999). This leaves the NH2-terminal PH domain and its embedded PDZ domain free to interact with other ligands and thereby link them to the dystrophin complex. Indeed, several molecules have been shown to bind these domains. The NH2-terminal PH domain can bind to phosphatidyl inositol-4,5-bisphosphate (Chockalingam et al., 1999). In vitro biochemical experiments have shown that the PDZ domain of α-syntrophin can bind to neuronal nitric oxide synthase (nNOS) (Brenman et al., 1996), muscle voltage-activated sodium channels (Gee et al., 1998a; Schultz et al., 1998), and stress-activated protein kinase-3 (Hasegawa et al., 1999). The β2-syntrophin PDZ domain binds to microtubule-associated serine/threonine kinase, and the related syntrophin-associated serine/threonine kinase (Lumeng et al., 1999). Diacylglycerol kinase ζ associates with the PDZ domain of γ1-syntrophin (Hogan et al., 2001). Discovery of these interactions has led to the proposal that syntrophins' function is to link signaling molecules to the dystrophin protein complex.
Generation and characterization of α-syntrophin–null mice have further strengthened this conclusion. Although the absence of α-syntrophin does not result in discernable musclular dystrophy (Kameya et al., 1999; Adams et al., 2000), the neuromuscular junctions of these mice are structurally aberrant, have reduced levels of acetylcholine receptors and acetylcholine esterase, and are missing utrophin, an ortholog of dystrophin (Adams et al., 2000). Furthermore, nNOS is lost from the sarcolemma in these mice, perhaps due to the absence of the syntrophin PDZ domain.
In addition to nNOS, the water channel, aquaporin-4, also shows dependence on α-syntrophin for sarcolemmal localization. Aquaporin-4 is absent from the sarcolemma when α-syntrophin is missing either due to the loss of the entire dystrophin complex in the mdx mouse (Frigeri et al., 1998) or specific loss of α-syntrophin by targeted gene disruption (Yokota et al., 2000). Aquaporins are responsible for the high membrane permeability of water in many different tissues, including kidney, brain, and the gastrointestinal tract (Frigeri et al., 1995). Aquaporin-4 is an integral membrane protein present in the sarcolemma of fast twitch fibers, although its physiological role in muscle is unknown. The COOH terminus of aquaporin-4 contains a likely PDZ domain interaction sequence, -VLSSV. However, this sequence is not one that we would expect a priori to bind syntrophin PDZs based on the published consensus binding sequences (Gee et al., 1998a, 1998b). Thus, the functional relationship between the α-syntrophin PDZ domain and sarcolemmal aquaporin-4 expression is unknown.
PDZ domains are involved in the assembly of macromolecular complexes at numerous sites of membrane specialization. These specializations include the neuromuscular synapse (Adams et al., 2000), synapses between neurons in the brain (Sheng, 1996; Kornau et al., 1997), the apical and basoloateral membranes of polarized epithelia (Short et al., 1998; Kachinsky et al., 1999), and receptor-dense areas that induce apoptosis in tumor cells (Yanagisawa et al., 1997). The most common mode of interaction is between the COOH-terminal tail of ligand and the PDZ domain (Doyle et al., 1996), although association with internal PDZ binding sequences and PDZ-PDZ heterodimerization also occurs (Gee et al., 1998b; Hillier et al., 1999; Tochio et al., 2000). Despite the fact that PDZ proteins have been studied extensively and the structure is known in great detail (Hillier et al., 1999), the PDZ–ligand interactions have not been characterized in vivo. An in vivo demonstration is important for two reasons. First, other interactions have been proposed to play a role in the localization of nNOS to the membrane by α-syntrophin. (Venema et al., 1997; Abdelmoity et al., 2000). The relative importance of these multiple binding sites has yet to be determined. Second, and perhaps more important, the large number of PDZ-containing proteins and potential binding proteins undoubtedly requires a level of specificity that extends beyond that achieved by the amino acid sequence adjacent to the terminal S-X-V motif. We observed such specificity with the PDZ domain of β1-syntrophin that can bind nNOS in vitro (Gee et al., 1998a), but that is unable to recruit nNOS to the sarcolemma in vivo in the absence of α-syntrophin (Adams et al., 2000). The development of in vivo systems for the study of this and similar phenomena will be very important in understanding how specificity of the formation of the complexes is governed, and to what extent the interactions found in vitro actually exist in the cell.
These issues have prompted us to develop mouse models to test the function of the α-syntrophin PDZ interactions in vivo. We have developed lines of transgenic mice that express a form of α-syntrophin in which the PDZ domain has been replaced with an epitope tag. We have used this transgenic line in combination with one expressing full-length α-syntrophin in dominant negative and genetic rescue experiments. These new mouse models demonstrate that nNOS and aquaporin-4, but not sodium channels, require the presence of an α-syntrophin PDZ domain for sarcolemmal localization in vivo. Furthermore, α-syntrophin requires the presence of the dystrophin complex to form a stable association with the sarcolemma.
Results
In vitro experiments have shown that the PDZ domain of α-syntrophin can associate with the sarcolemmal proteins nNOS (Brenman et al., 1996) and the voltage gated sodium channels, SCN4A and SCN5A (Gee et al., 1998a; Schultz et al., 1998). To address the role of the syntrophin PDZ domain in vivo, we developed transgenic mice expressing a modified α-syntrophin lacking the PDZ domain.
Generation and characterization of transgenic mice
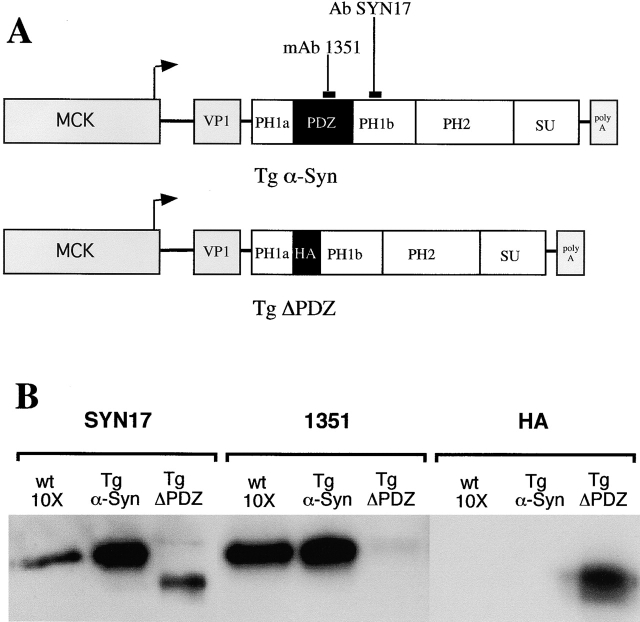
We developed two lines of transgenic mice (Fig. 1). The first, Tg α-Syn, expresses full-length α-syntrophin. The second, Tg ΔPDZ, expresses an altered form of α-syntrophin in which the PDZ domain was replaced with the hemagglutinin (HA) epitope tag. The expression of both proteins is under control of the mouse muscle creatine kinase promoter; therefore, they are expressed primarily in skeletal and cardiac muscle. Endogenously produced α-syntrophin can be differentiated from the ΔPDZ transgene product by the use of a monoclonal antibody (1351) that recognizes an epitope within the PDZ domain (Materials and methods). Both transgene products and endogenous α-syntrophin are recognized by rabbit polyclonal antibody, SYN17, as the antibody was made to a peptide sequence that lies just downstream of the PDZ domain. Immunoblot analysis using these antibodies shows that proteins derived from the transgenes are expressed at levels 20–30-fold higher than endogenous α-syntrophin in skeletal muscle (Fig. 1 B).
Figure 1.
Construction and characterization of transgenic mice. (A) The mouse cDNA sequence of full-length α-syntrophin and α-syntrophin missing the PDZ domain (ΔPDZ) was placed downstream of the mouse muscle creatine kinase (MCK) promoter and the VP1 intron of plasmid pCKVA. The PDZ domain of the Tg ΔPDZ construct was replaced with an HA tag. The binding sites for mAb 1351 and Ab SYN 17 are shown. (B) Expression of transgene products was examined by immunoblotting. 20 μg (wild-type control) and 2 μg (transgenics) of total protein isolated from skeletal muscle were subjected to immunoblot analysis. SYN17 recognizes both endogenous and transgenic α-syntrophin; mAb 1351 recognizes endogenous and Tg α-Syn but not Tg ΔPDZ; HA recognizes only Tg ΔPDZ. Very small amounts of endogenous α-syntrophin can be seen in the Tg ΔPDZ preparations with SYN17 and mAb 1351.
We characterized the distribution of the transgene products within muscle by immunofluorescence (Fig. 2). Mouse quadriceps muscle was labeled using the same set of antibodies used for the Western blots in Fig. 1. Labeling with the anti-HA antibody shows that the ΔPDZ transgene product accumulates on the sarcolemma. Cytosolic labeling can be seen for both transgene products and is most likely due to the high levels of expression of these proteins, which cannot be accommodated by the available membrane binding sites.
Figure 2.
Comparative localization of α-syntrophin in control and transgenic mice. Immunofluorescence microscopy of adult (>8 wk) mouse quadriceps muscle shows that the HA-tagged Tg ΔPDZ transgene product is localized at the sarcolemma. Labeling with mAb 1351 is strong on normal and Tg α-Syn muscle but shows greatly reduced intensity in the Tg ΔPDZ muscle. Thus, Tg ΔPDZ syntrophin displaces the endogenous α-syntrophin. Antibody SYN17 that recognizes both transgene products and endogenous α-syntrophin shows a modest increase in labeling intensity at the sarcolemma and in the cytosol in the transgenic muscle. β1-Syntrophin is displaced by the high expression of the Tg ΔPDZ product but remains present, although at reduced levels, in the muscle of the full-length α-syntrophin transgenic mouse. β1-Syntrophin is also detected in blood vessels surrounding muscle fibers in all sections. Bar, 50 μm.
Labeling with antibody 1351 is greatly reduced in the Tg ΔPDZ muscle (Fig. 2). Sarcolemmal labeling is faint or absent (Fig. 2) although neuromuscular junctions remain brightly labeled (unpublished data). These results indicate that the endogenous α-syntrophin is displaced from the sarcolemma by the high levels of ΔPDZ-syntrophin.
Tg ΔPDZ protein also displaces β1-syntrophin (Fig. 2). Sarcolemmal labeling for β1-syntrophin is greatly reduced in the Tg ΔPDZ muscle, but neighboring blood vessels, where the transgene is not expressed, show normal high levels of expression. The Tg α-Syn protein does not displace β1-syntrophin to the same extent as the Tg-ΔPDZ protein, despite being expressed at somewhat higher levels (Fig 1 B). Sarcolemmal levels of β1-syntrophin are reduced in the muscles of the Tg α-Syn mice, but are still clearly present and even brightly labeled on the smaller fibers. These small fibers may represent areas of immature muscle. β1-Syntrophin is expressed in most skeletal muscle fibers during the first six weeks of mouse muscle development after which its sarcolemma levels decline in all but the fast type II fibers (Kramarcy and Sealock, 2000). Other than having a higher number of small fibers, muscles of the transgenic mice did not show any of the abnormalities commonly found in dystrophic muscle (central nuclei, split fibers, fibrosis, etc.).
Association of transgenic syntrophins with dystrophin
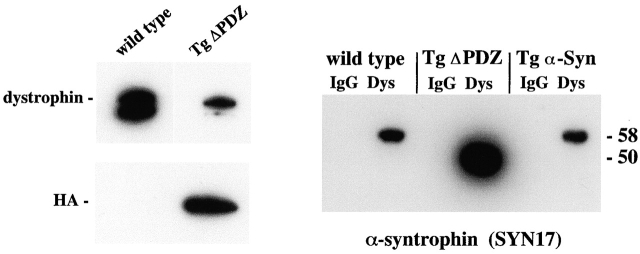
Syntrophin associates with the sarcolemma by binding directly to dystrophin and dystrobrevin (Ahn and Kunkel, 1995, Dwyer and Froehner, 1995, Yang et al., 1995; Newey et al., 2000). To determine if the transgenic syntrophins Tg ΔPDZ and Tg α-Syn bound dystrophin, we isolated the dystrophin complex from muscle homogenates by immunoprecipitation with anti-dystrophin antibody. Immunoblotting of the complexes with anti-HA antibody showed that the Tg ΔPDZ protein copurified with dystrophin (Fig. 3). Similar blotting with SYN17 confirmed the presence of Tg ΔPDZ and also showed that Tg α-Syn copurified with dystrophin as expected (Fig. 3). We consistently observed proportionally more Tg ΔPDZ than either the endogenous or transgenic full-length syntrophin in the dystrophin isolates (Fig. 3), suggesting that the Tg ΔPDZ syntrophin may have a higher affinity for dystrophin than the full-length forms. This conclusion is consistent with the immunofluorescence data (Fig. 2) showing that Tg ΔPDZ syntrophin displaced β1-syntrophin to a greater extent than did Tg α-Syn.
Figure 3.
Association of the Tg ΔPDZ product with dystrophin. Dystrophin and associated proteins were immunoprecipitated from wild-type (C57) and Tg ΔPDZ skeletal muscle (Dys). Control samples were prepared by substituting normal mouse IgG for the dystrophin antibody (IgG). (A) Immunoblotting with the HA antibody shows that the transgene product copurifies with dystrophin. (B) Immunoblotting with SYN17 confirms that the Tg ΔPDZ product displaces endogenous syntrophin from the dystrophin complex (no 58-kD band, corresponding to the full-length endogenous α-syntrophin, is seen in the Tg ΔPDZ lane).
Distribution of syntrophin PDZ ligands in transgenic skeletal muscle
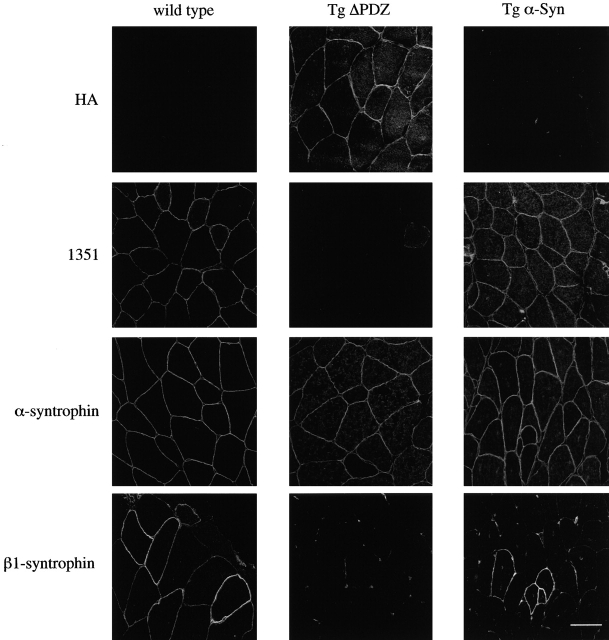
Having shown that the Tg ΔPDZ syntrophin displaces endogenous α- and β1-syntrophin, we examined how the absence of a sarcolemmal syntrophin PDZ domain affected the distribution of PDZ ligands. The distributions of nNOS, sodium channels, and aquaporin-4 were examined by immunofluorescence microscopy (Fig. 4). In the absence of a sarcolemmal α-syntrophin PDZ domain, nNOS is no longer present on the sarcolemma. Thus, the syntrophin PDZ domain is necessary for localization of nNOS to the sarcolemma in a stable manner.
Figure 4.
Immunofluorescence analysis of syntrophin-associated proteins in mouse quadriceps muscle. In the absence of the α-syntrophin PDZ domain (Tg ΔPDZ) or dystrophin (mdx), nNOS and aquaporin-4 fail to localize at the sarcolemma while sodium channel (NaCh) distribution appears unchanged. Dystrophin levels appear normal in the transgenic animals. Note: the dystrophin-labeled fiber in the mdx mouse is probably a revertant fiber and serves as a convenient focussing and antibody control. Bar, 50 μm.
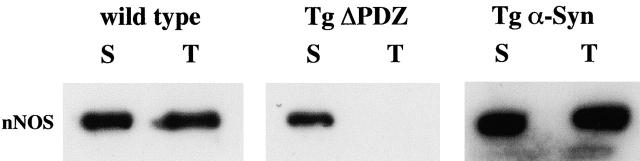
In wild-type skeletal muscle, nNOS resides both in the cytosol and at the sarcolemma where it is linked to the cytoskeleton via the syntrophin/dystrophin complex. To confirm the immunofluorescence data, we determined the relative levels of nNOS in the soluble (cytosolic) and detergent-soluble (membrane associated) fractions of mouse muscle (Fig. 5). In both wild-type and Tg α-Syn mice, nNOS was present in both fractions. However, in samples from Tg ΔPDZ mice, nNOS was present only in the soluble fraction supporting the conclusion that the sarcolemmal localization of nNOS requires the α-syntrophin PDZ domain.
Figure 5.
nNOS is shifted from cytoskeletal association to the soluble pool in the Tg ΔPDZ muscle. Samples from soluble cytosolic (S) or 1% Triton (T) skeletal muscle extracts were analyzed for nNOS by immunoblotting. In the absence of the α-syntrophin PDZ domain, nNOS is found only in the soluble fraction. High levels of expression of full-length α-syntrophin do not change the relative amounts of nNOS in the cytosolic and insoluble fractions.
Previous reports have implicated the dystrophin complex (Frigeri et al., 1998) and α-syntrophin (Yokota et al., 2000) in the expression of aquaporin-4 at the plasma membrane of fast twitch muscle. To determine the importance of the PDZ domain in aquaporin-4 sarcolemmal expression, we examined the distribution of aquaporin-4 in the α-syntrophin transgenic mice. As shown in Fig. 4, aquaporin-4 is no longer present at the sarcolemma in the absence of a syntrophin PDZ domain. The loss of sarcolemmal aquaporin-4 occurs even though the distribution of dystrophin is unaffected in the transgenic mice (Fig. 4). Unlike nNOS, which associates peripherally with the sarcolemma, aquaporin-4 is an integral membrane protein. Its absence from the sarcolemma of Tg ΔPDZ mouse muscle implies that syntrophin must function to target or stabilize aquaporin-4 in muscle. Conversely, the absence of a syntrophin PDZ domain had no apparent affect on the distribution or level of expression of voltage gated sodium channels in muscle (Fig. 4).
PDZ ligand restoration in syntrophin-null/transgenic crosses
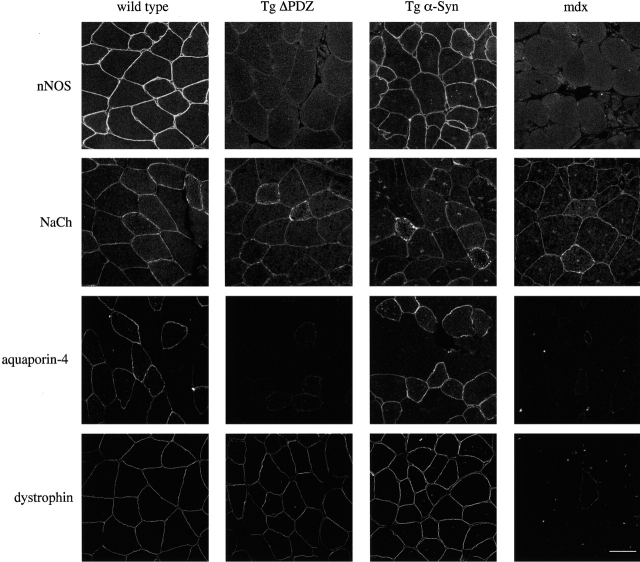
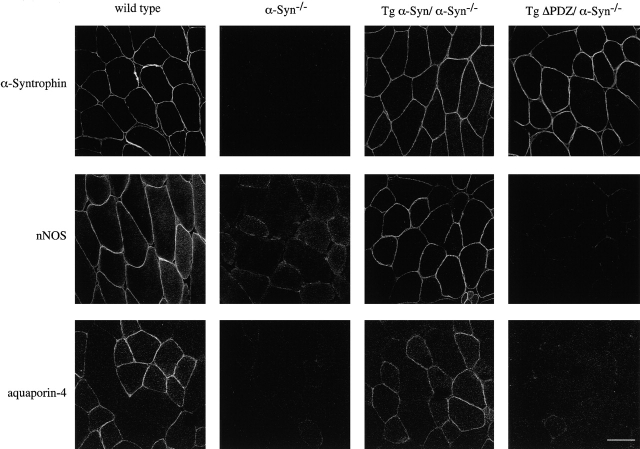
In genetically modified mice lacking α-syntrophin, nNOS is absent from the sarcolemma (Kameya et al., 1999; Adams et al., 2000). This has been assumed to be due to the loss of the α-syntrophin PDZ domain. We tested this assumption directly by breeding the two syntrophin transgenic mice lines onto the α-syntrophin–null background. Immunofluorescence studies show that the sarcolemmal localization of nNOS is “rescued” by the full-length syntrophin transgene but not by the ΔPDZ transgene (Fig. 6). Thus, in vivo, nNOS localization to the sarcolemma depends on the presence of an α-syntrophin PDZ domain. Similarly, aquaporin-4, normally absent from the sarcolemma of α-syntrophin–null mice, was restored by the full-length transgene but not by the ΔPDZ construct (Fig. 6). Our attempts to show in vitro that the aquaporin-4 COOH-terminal sequence binds directly to α-syntrophin have been unsuccessful, even under conditions in which a similar peptide corresponding to the COOH terminus of the sodium channel binds robustly (unpublished data). Nevertheless, these in vivo data clearly show that sarcolemmal localization of aquaporin-4 requires the α-syntrophin PDZ domain.
Figure 6.
Sarcolemmal expression of nNOS and aquaporin-4 in genetic crosses of α-syntrophin–null mice with transgenic mice. Both nNOS and aquaporin-4 are absent from the sarcolemma of α-syntrophin–null mice. Muscle from α-syntrophin–null mice crossed with Tg α-Syn mice shows sarcolemmal expression of both nNOS and aquaporin-4. Sarcolemmal expression of these proteins is not restored in muscle of α-syntrophin–null mice crossed with Tg ΔPDZ mice. Bar, 50 μm.
Syntrophin transgene localization in dystrophin-deficient muscle
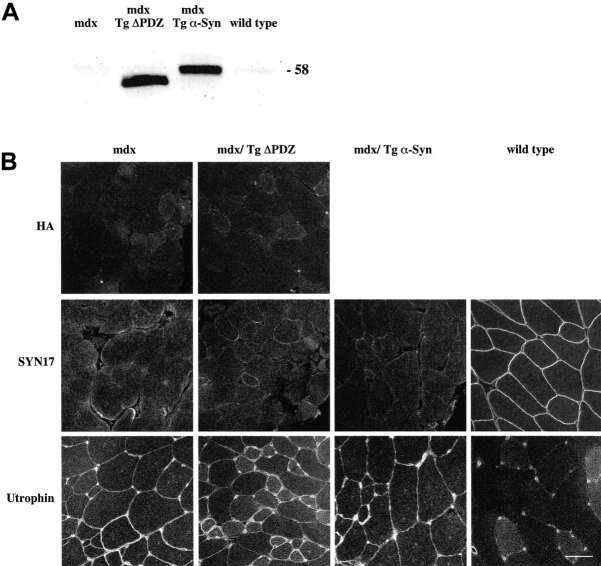
Both the Tg ΔPDZ and the Tg α-Syn mouse lines were bred with the mdx mice to produce mice overexpressing syntrophin in the absence of dystrophin. Immunofluorescence analysis of the muscles of these mice showed that despite being expressed at high levels (Fig. 7 A), neither protein associated with the sarcolemma in the absence of dystrophin (Fig. 7 B). Histologically, the Tg ΔPDZ/mdx and the Tg α-Syn/mdx muscles were indistinguishable from mdx controls (unpublished data). It is noteworthy that even though low levels of utrophin are expressed on the mdx sarcolemma, we did not detect sarcolemmal α-syntrophin (Fig. 7). Thus, α-syntrophin may have a relatively low affinity for utrophin, consistent with previous immunoisolation experiments (Peters et al., 1997). The PH domain association with phosphatidyl inositol-4,5-bisphosphate (Chockalingam et al., 1999) and the PDZ domain association with the COOH-terminal tail of sodium channels (Gee et al., 1998a; Schultz et al., 1998) are apparently insufficient to recruit α-syntrophin to the sarcolemma in the absence of dystrophin.
Figure 7.
Distribution of Tg ΔPDZ and Tg α-Syn in the absence of dystrophin. (A) Both Tg ΔPDZ and Tg α-Syn proteins (detected with SYN17) are expressed at high levels in muscle lacking dystrophin (mdx/Tg ΔPDZ and mdx/Tg α-Syn). (B) Despite high levels of transgene product expression, neither the full-length nor the Tg ΔPDZ syntrophin are found on the sarcolemma. Utrophin sarcolemmal expression is upregulated in the mdx mouse but apparently to levels insufficient to recruit α-syntrophin. Bar, 50 μm.
Discussion
The role of PDZ domain proteins in targeting, anchoring, and stabilizing membrane channels and receptors has been investigated using a variety of approaches such as peptide overlays, yeast two-hybrid assays, surface plasmon resonance, and cell transfection experiments. Many PDZ-ligand interactions have been well characterized in vitro, including AMPA receptors, which interact with the PDZ domain proteins GRIP (Dong et al., 1997) and PICK-1 (Xia et al., 1999); the cystic fibrosis transmembrane receptor, which binds the PDZ domain proteins CAP-70 (CFTR-associated protein, 70 kDA) (Wang et al., 2000) and the Na+/H+ exchanger regulatory factor (Raghuram et al., 2001); and the PDZ domains of postsynaptic density protein-95 and other members of the MAGUK family of proteins, which bind to NMDA receptor (Kornau et al., 1995) and to nNOS (Brenman et al., 1996). However, recent findings from our lab suggest that at least in some cases PDZ-ligand interactions that occur in vitro may not occur in vivo. The PDZ domains of α, β1, and β2 syntrophin have been shown to bind nNOS with similar affinity in vitro (Gee et al., 1998b), but in skeletal muscle of the α-syntrophin–null mouse β1 and β2 syntrophin do not associate with nNOS (Adams et al., 2000). The demonstration of in vivo specificity of PDZ domains has prompted us to use in vivo approaches to investigate the physiological relevance of PDZ domain/ligand interactions.
Some PDZ-ligands have been characterized in vivo using invertebrate models. In Caenorhabditis elegans, three PDZ proteins, Lin-2, Lin-7, and Lin-10, mediate the localization of the LET-23 receptor (Kaech et al., 1998). This receptor fails to localize to the basolateral surface when the PDZ proteins are mutated. Mutational studies in Drosophila show that the PDZ domain–containing protein, discs large, is required for the neuromuscular junction localization of Shaker potassium channels (Tejedor et al., 1997). However, no direct test of PDZ-ligand interactions has been reported in whole animal mammalian systems. In this paper, we have described one approach that should be generally applicable to in vivo analyses in a broad range of systems.
We investigated the functional role of the α-syntrophin PDZ domain in vivo by using a dominant-negative approach. We developed a transgenic mouse line that expresses a PDZ-less form of α-syntrophin at high levels in skeletal muscle. The observation that ΔPDZ α-syntrophin is localized to the sarcolemma indicates that the PDZ domain is not required for plasma membrane association. This result is consistent with the demonstration that the PH2 and SU domains in tandem are required for binding to dystrophin and other family members (Ahn and Kunkel, 1995; Kachinsky et al., 1999). Furthermore, the ΔPDZ form of α-syntrophin competed with endogenous α-syntrophin for binding sites on the sarcolemma (dystrophin and dystrobrevin). This competition produced skeletal muscle that contained little or no sarcolemmal syntrophin PDZ domain as demonstrated by the reduction in labeling with mAb 1351, an antibody that binds to an epitope within the PDZ domain of α-syntrophin.
In addition to displacing endogenous α-syntrophin, the ΔPDZ α-syntrophin was able to displace β1-syntrophin from the sarcolemma as well. These data suggest that α- and β1-syntrophin compete for the same binding sites, at least under conditions of high α-syntrophin expression, to facilitate membrane association. Interestingly, the ΔPDZ form was more effective than the full-length Tg α-syntrophin in displacing β1-syntrophin. This suggests that the absence of the PDZ domain increases the affinity of syntrophin for dystrophin/dystrobrevin. Thus, the PDZ domain of syntrophin may act to regulate the interaction of syntrophin with the dystrophin complex. An interesting possibility is that binding of a ligand to the PDZ domain promotes association of the PH-SU domains with their binding sites on dystrophin. Such regulation is similar to that demonstrated for MAGUK family proteins (McGee and Bredt, 1999) and could be important for localizing PDZ ligands to the sarcolemma via the dystrophin complex.
Two syntrophin PDZ ligands have been studied extensively in vitro. nNOS and voltage-gated sodium channels bind to α-syntrophin PDZ domains but via different mechanisms. nNOS contains a beta-finger immediately adjacent to its own PDZ domain that interacts directly with the PDZ domain of syntrophin (Hillier et al., 1999). In contrast, sodium channels bind to syntrophin PDZ domains via the more conventional COOH terminal “SXV” motif (Gee et al., 1998a). The function of this interaction is unknown, but syntrophin binding could potentially affect the ion conduction properties of the sodium channel or mediate its participation in a scaffold involving other channels and/or modifying enzymes. However, our data show that syntrophin binding is not required for normal sodium channel distribution on the sarcolemma. This conclusion is in agreement with the observation that sodium channel density is only slightly reduced in skeletal muscle of the mdx mouse which lacks dystrophin and, therefore, sarcolemmal syntrophin (Ribaux et al., 2001).
In contrast, sarcolemmal localization of nNOS does depend on the presence of an α-syntrophin PDZ domain. In the absence of a sarcolemmal syntrophin PDZ domain, nNOS fails to localize to the sarcolemma. The localization of nNOS to the sarcolemma is important in regulating blood flow in skeletal muscle during muscle activity. Thomas et al. (1998) have shown that in the absence of nNOS, attenuation of adrenergically stimulated vasoconstriction does not occur. Similar results were obtained from mdx mice which lack sarcolemmal nNOS (Thomas et al., 1998). Therefore, it is likely that the α-syntrophin PDZ domain is not only necessary for proper localization of nNOS but is required for proper function of nNOS in skeletal muscle as well.
In addition to syntrophin, another membrane-associated protein, caveolin-3, has been shown to bind nNOS (Venema et al., 1997). However, in muscle from the Tg ΔPDZ mice and the Tg ΔPDZ/ α-syntrophin–null cross, we could not detect nNOS on the membrane. Therefore, the interaction of nNOS with caveolin-3 must be insufficient for stable retention of nNOS at the sarcolemma. It has also been proposed that nNOS may localize to the sarcolemma by binding membrane-associated proteins directly via its PDZ domain (Abdelmoity et al., 2000). Because in the absence of a syntrophin PDZ domain we do not detect nNOS on the sarcolemma, if such interactions occur they are insufficient for stable retention. Alternatively, such interactions could be induced by association of the nNOS beta finger with the syntrophin PDZ domain.
A third putative ligand for the α-syntrophin PDZ domain is aquaporin-4. Aquaporin-4 is normally present on the sarcolemma of fast twitch fibers of skeletal muscle but is absent from muscle that lacks the dystrophin protein complex (Frigeri et al., 1998). The COOH-terminal amino acid sequence of aquaporin-4 is -VLSSV, a potential class I PDZ domain interaction sequence. Therefore, we used our transgenic mice to determine if the membrane localization of aquaporin-4 depended on a syntrophin PDZ domain. In the absence of an α-syntrophin PDZ domain, aquaporin-4 is also absent from the sarcolemma. Aquaporin-4 is an integral membrane protein that contains six transmembrane domains and therefore does not need to be recruited to the sarcolemma like the soluble protein, nNOS. Rather, the syntrophin PDZ domain is likely involved in targeting or stabilizing aquaporin-4.
The in vivo studies presented here also provide insight into how α-syntrophin interacts with the sarcolemma. Data from the Tg ΔPDZ show that α-syntrophin is localized to the sarcolemma and binds directly to dystrophin in the absence of a PDZ domain. However, the immunofluorescence studies of muscle expressing full-length and ΔPDZ syntrophin on an mdx background show that, even when expressed at high levels, syntrophin does not associate with the sarcolemma in the absence of dystrophin. Apparently, interactions with sodium channels or phosphatidyl inositols are insufficient to localize syntrophin to the sarcolemma. In fact, our data indicate that interaction with the dystrophin complex is necessary and sufficient for sarcolemmal α-syntrophin localization.
Materials and methods
Generation of transgenic mice
We have previously isolated and characterized the mouse cDNA encoding α-syntrophin (Adams et al., 1993). This cDNA was modified by excising nucleotides 332–622 with restriction enzymes Ec47 and EcoRI (New England Biolabs) to remove the sequence encoding 98 amino acids of the PDZ domain. Excised sequence was replaced with a double stranded synthetic oligonucleotide (Macromolecular Resources) encoding the HA epitope. The resulting construct, designated ΔPDZ, and the full-length α-syntrophin cDNA were cloned into the SacII site of vector pCKVA (Cox et al., 1993), a gift from Jeff Chamberlain (University of Washington, Seattle, WA). The pCKVA vector contains 3,300 bp of the mouse muscle creatine kinase promoter, a 700-bp viral intron, and a 200-bp poly-A signal sequence. The linearized constructs were injected into C57Bl6 oocytes (University of North Carolina Transgenic Facility, Chapel Hill, NC). Three independent lines of mice harboring the ΔPDZ construct (Tg ΔPDZ) and nine lines of mice harboring the full-length construct (Tg α-Syn) were identified by Southern blotting and PCR.
Mice expressing Tg ΔPDZ and mice expressing Tg α-Syn were bred onto an α-syntrophin–null background by crossing with the previously characterized α-syntrophin–null mice (Adams et al., 2000). Similarly, the transgenic lines were bred onto the mdx background. In each case the genotypes were confirmed by PCR of genomic DNA derived from tail biopsies.
Antibodies
Isoform specific rabbit polyclonal antibodies SYN17 (α-syntrophin) and SYN37 (β1-syntrophin) have been previously characterized (Peters et al., 1997). The polyclonal anti-HA antibody was purchased from Zymed Laboratories, Inc. For detection of nNOS, two antibodies were used: an mAb from BD Transduction Laboratories (San Diego, CA) was used for Western blots, and a polyclonal antibody (Ab) from Diasorin (Stillwater, MN) was used for immunofluorescence microscopy. The polyclonal Ab to aquaporin-4 was purchased from Chemicon. The polyclonal Ab recognizing both the SCN4A and SCN5A isoforms of the sodium channel was a gift from S. Rock Levinson, (University of Colorado Health Sciences Center, Denver, CO). The utrophin polyclonal Ab was raised against a peptide at the COOH terminus identical to that previously described for Ab 1862 (Kramarcy et al., 1994). The mAb Mandra 1, recognizing dystrophin, was purchased from Sigma-Aldrich.
We have previously described mAb 1351 (Froehner et al., 1987) and have shown that it recognizes an epitope within the PDZ domain of α-, β1-, and β2-syntrophin (Gee et al., 1998a). However, for this study it was important to identify the epitope more specifically. Therefore, we synthesized 29 overlapping peptides of 13 amino acids each that spanned the entire PDZ domain of α-syntrophin (SPOTs blot; Genosys). Incubation of mAb 1351 with these peptides using the protocol supplied by the manufacturer showed that the epitope is confined to a 13–amino acid segment with the sequence: ISKIFKGLAADQT. This sequence corresponds to amino acids 108–120 within the α-syntrophin PDZ domain.
Immunoblotting
Muscle protein detergent extracts were prepared as previously described (Peters et al., 1997). Dystrophin was isolated by incubating muscle extracts with the dystrophin mAb Mandra-1 followed by precipitation with protein G–coated beads (Sigma-Aldrich). After washing the beads three times with phosphate buffered saline (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4), we resuspended the specifically bound protein in SDS-PAGE sample buffer. Protein samples were subjected to electrophoresis on an 8% polyacrylamide tricine-buffered gel. Separated proteins were transferred to Immobilon-P membrane (Millipore Corp.) and incubated with the indicated antibody as previously described (Peters et al., 1997). Bands were visualized using a peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) and a luminol-based substrate (Pierce Chemical Co.).
Fluorescence microscopy
Immunofluorescence labeling was performed on unfixed muscle flash frozen in liquid nitrogen cooled isopentane. 8-μm cryosections were incubated with antibody (30 nM IgG, in each case) as described by Peters et al. (1997). Primary antibodies were visualized using Alexa-488 and -568 (Molecular Probes) conjugated secondary antibodies. Sections were analyzed using a Leica TCS NT confocal microscope.
Acknowledgments
We thank S. Rock Levinson for sodium channel antibody, Jeff Chamberlain for pCKVA vector, L. Gretta Gray (University of North Carolina, Chapel Hill, North Carolina) for mouse management, Amy Alessi (Harvard University, Boston, MA) for initiating the aquaporin-4 experiments, and our colleagues in the lab for helpful discussions and critical reading of the manuscript.
This work was funded by grants from the Muscular Dystrophy Association (M.E. Adams and S.C. Froehner) and from the National Institutes of Health (NS33145).
H. Mueller's present address is Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599.
Footnotes
Abbreviations used in this paper: Ab, antibody; HA, hemagglutinin; nNOS, neuronal nitric oxide synthase; PDZ, PSD-95, discs-large, ZO-1; PH, pleckstrin homology; SU, syntrophin unique region.
References
- Abdelmoity, A., R.C. Padre, K.E. Burzynski, J.T. Stull, and K.S. Lau. 2000. Neuronal nitric oxide synthase localizes through multiple structural motifs to the sarcolemma in mouse myotubes. FEBS Lett. 482:65–70. [DOI] [PubMed] [Google Scholar]
- Adams, M.E., M.H. Butler, T.M. Dwyer, M.F. Peters, A.A. Murnane, and S.C. Froehner. 1993. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 11:531–540. [DOI] [PubMed] [Google Scholar]
- Adams, M.E., T.M. Dwyer, L.L. Dowler, R.A. White, and S.C. Froehner. 1995. Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J. Biol. Chem. 270:25859–25865. [DOI] [PubMed] [Google Scholar]
- Adams, M.E., N. Kramarcy, S.P. Krall, S.G. Rossi, R.L. Rotundo, R. Sealock, and S.C. Froehner. 2000. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J. Cell Biol. 150:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, A.H., and L.M. Kunkel. 1995. Syntrophin binds to an alternatively spliced exon of dystrophin. J. Cell Biol. 128:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, A.H., M. Yoshida, M.S. Anderson, C.A. Feener, S. Selig, Y. Hagiwara, E. Ozawa, and L.M. Kunkel. 1994. Cloning of human basic A1, a distinct 59-kDa dystrophin-associated protein encoded on chromosome 8q23-24. Proc. Natl. Acad. Sci. USA. 91:4446–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman, J.E., D.S. Chao, S.H. Gee, A.W. McGee, S.E. Craven, D.R. Santillano, Z. Wu, F. Huang, H. Xia, M.F. Peters, S.C. Froehner, and D.S. Bredt. 1996. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 84:757–767. [DOI] [PubMed] [Google Scholar]
- Butler, M.H., K. Douville, A.A. Murnane, N.R. Kramarcy, J.B. Cohen, R. Sealock, and S.C. Froehner. 1992. Association of the Mr 58,000 postsynaptic protein of electric tissue with Torpedo dystrophin and the Mr 87,000 postsynaptic protein. J. Biol. Chem. 267:6213–6218. [PubMed] [Google Scholar]
- Chockalingam, P.S., S.H. Gee, and H.W. Jarrett. 1999. Pleckstrin homology domain 1 of mouse alpha1-syntrophin binds phosphatidylinositol 4,5-bisphosphate. Biochemistry. 38:5596–5602. [DOI] [PubMed] [Google Scholar]
- Cox, G.A., N.M. Cole, K. Matsumura, S.F. Phelps, S.D. Hauschka, K.P. Campbell, J.A. Faulkner, and J.S. Chamberlain. 1993. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 364:725–729. [DOI] [PubMed] [Google Scholar]
- Dong, H., R.J. O'Brien, E.T. Fung, A.A. Lanahan, P.F. Worley, and R.L. Huganir. 1997. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 386:279–284. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., A. Lee, J. Lewis, E. Kim, M. Sheng, and R. MacKinnon. 1996. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 85:1067–1076. [DOI] [PubMed] [Google Scholar]
- Dwyer, T.M., and S.C. Froehner. 1995. Direct binding of Torpedo syntrophin to dystrophin and the 87 kDa dystrophin homologue. FEBS Lett. 375:91–94. [DOI] [PubMed] [Google Scholar]
- Frigeri, A., M.A. Gropper, C.W. Turck, and A.S. Verkman. 1995. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA. 92:4328–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri, A., G.P. Nicchia, J.M. Verbavatz, G. Valenti, and M. Svelto. 1998. Expression of aquaporin-4 in fast twitch fibers of mammalian skeletal muscle. J. Clin. Invest. 102:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner, S.C., A.A. Murnane, M. Tobler, H.B. Peng, and R. Sealock. 1987. A postsynaptic Mr 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J. Cell Biol. 104:1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., R. Madhavan, S.R. Levinson, J.H. Caldwell, R. Sealock, and S.C. Froehner. 1998. a. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci. 18:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., S.A. Sekely, C. Lombardo, A. Kurakin, S.C. Froehner, and B.K. Kay. 1998. b. Cyclic peptides as non-carboxy-terminal ligands of syntrophin PDZ domains. J. Biol. Chem. 273:21980–21987. [DOI] [PubMed] [Google Scholar]
- Hasegawa, M., A. Cuenda, M.G. Spillantini, G.M. Thomas, V. Buee-Scherrer, P. Cohen, and M. Goedert. 1999. Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J. Biol. Chem. 274:12626–12631. [DOI] [PubMed] [Google Scholar]
- Hillier, B.J., K.S. Christopherson, K.E. Prehoda, D.S. Bredt, and W.A. Lim. 1999. Unexpected modes of PDZ scaffolding revealed by structure of nNOS-syntrophin complex. Science. 284:812–815. [PubMed] [Google Scholar]
- Hoffman, E.P., R.H. Brown, and L.M. Kunkel. 1987. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 51:919–928. [DOI] [PubMed] [Google Scholar]
- Hogan, A., L. Shepherd, J. Chabot, S. Quenneville, S.M. Prescott, M.K. Topham, and S.H. Gee. 2001. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta: Regulation of nuclear localization by PDZ interactions. J. Biol. Chem. 276:26526–26533. [DOI] [PubMed] [Google Scholar]
- Kachinsky, A.M., S.C. Froehner, and S.L. Milgram. 1999. A PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells. J. Cell Biol. 145:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech, S.M., C.W. Whitfield, and S.K. Kim. 1998. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 94:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameya, S., Y. Miyagoe, I. Nonaka, T. Ikemoto, M. Endo, K. Hanaoka, Y. Nabeshima, and S. Takeda. 1999. Alpha-syntrophin gene disruption results in the absence of neuronal-type nitric oxide synthase at the sarcolemma but does not induce muscle degeneration. J. Biol. Chem. 274:2193–2200. [DOI] [PubMed] [Google Scholar]
- Kornau, H.C., L.T. Schenker, M.B. Kennedy, and P.H. Seeburg. 1995. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 269:1737–1740. [DOI] [PubMed] [Google Scholar]
- Kornau, H.C., P.H. Seeburg, and M.B. Kennedy. 1997. Interaction of ion channels and receptors with PDZ domain proteins. Curr. Opin. Neurobiol. 7:368–373. [DOI] [PubMed] [Google Scholar]
- Kramarcy, N.R., and R. Sealock. 2000. Syntrophin isoforms at the neuromuscular junction: Developmental time course and differential localization. Mol. Cell. Neurosci. 15:262–274. [DOI] [PubMed] [Google Scholar]
- Kramarcy, N.R., A. Vidal, S.C. Froehner, and R. Sealock. 1994. Association of utrophin and multiple dystrophin short forms with the mammalian Mr 58,000 dystrophin-associated protein (syntrophin). J. Biol. Chem. 269:2870–2876. [PubMed] [Google Scholar]
- Lumeng, C., S. Phelps, G.E. Crawford, P.D. Walden, K. Barald, and J.S. Chamberlain. 1999. Interactions between beta2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat. Neurosci. 2:611–617. [DOI] [PubMed] [Google Scholar]
- McGee, A.W., and D.S. Bredt. 1999. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J. Biol. Chem. 274:17431–17436. [DOI] [PubMed] [Google Scholar]
- Newey, S.E., M.A. Benson, C.P. Ponting, K.E. Davies, and D.J. Blake. 2000. Alternative splicing of dystrobrevin regulates the stoichiometry of syntrophin binding to the dystrophin protein complex. Curr. Biol. 10:1295–1298. [DOI] [PubMed] [Google Scholar]
- Peters, M.F., M.E. Adams, and S.C. Froehner. 1997. Differential association of syntrophin pairs with the dystrophin complex. J. Cell Biol. 138:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M.F., N.R. Kramarcy, R. Sealock, and S.C. Froehner. 1994. b2-Syntrophin: Localization at the neuromuscular junction in skeletal muscle. Neuro. Report. 5:1577–1580. [PubMed] [Google Scholar]
- Peters, M.F., H. Sadoulet-Puccio, R.M. Grady, N.R. Kramarcy, L.M. Kunkel, J.R. Sanes, R. Sealock, and S.C. Froehner. 1998. Differential membrane localization and intermolecular associations of alpha-dystrobrevin isoforms in skeletal muscle. J. Cell Biol. 142:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piluso, G., M. Mirabella, E. Ricci, A. Belsito, C. Abbondanza, S. Servidei, A.A. Puca, P. Tonali, G.A. Puca and V. Nigro. 2000. Gamma1- and gamma2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J. Biol. Chem. 275:15851–15860. [DOI] [PubMed] [Google Scholar]
- Raghuram, V., D.D. Mak, and J.K. Foskett. 2001. Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction. Proc. Natl. Acad. Sci. USA. 98:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaux, P., F. Bleicher, M.L. Couble, J. Amsellem, S.A. Cohen, C. Berthier, and S. Blaineau. 2001. Voltage-gated sodium channel (SkM1) content in dystrophin-deficient muscle. Pflugers Arch. 441:746–755. [DOI] [PubMed] [Google Scholar]
- Sadoulet-Puccio, H.M., T.S. Khurana, J.B. Cohen, and L.M. Kunkel. 1996. Cloning and characterization of the human homologue of a dystrophin related phosphoprotein found at the Torpedo electric organ post-synaptic membrane. Hum. Mol. Genet. 5:489–496. [DOI] [PubMed] [Google Scholar]
- Schultz, J., U. Hoffmuller, G. Krause, J. Ashurst, M.J. Macias, P. Schmieder, J. Schneider-Mergener, and H. Oschkinat. 1998. Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nat. Struct. Biol. 5:19–24. [DOI] [PubMed] [Google Scholar]
- Sheng, M. 1996. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 17:575–578. [DOI] [PubMed] [Google Scholar]
- Short, D.B., K.W. Trotter, D. Reczek, S.M. Kreda, A. Bretscher, R.C. Boucher, M.J. Stutts, and S.L. Milgram. 1998. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273:19797–19801. [DOI] [PubMed] [Google Scholar]
- Tejedor, F.J., A. Bokhari, O. Rogero, M. Gorczyca, J. Zhang, E. Kim, M. Sheng, and V. Budnik. 1997. Essential role for dlg in synaptic clustering of Shaker K1 channels in vivo. J. Neurosci. 17:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G.D., M. Sander, K.S. Lau, P.L. Huang, J.T. Stull, and R.G. Victor. 1998. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl. Acad. Sci. USA. 95:15090–15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochio, H., Y.K. Mok, Q. Zhang, H.M. Kan, D.S. Bredt, and M. Zhang. 2000. Formation of nNOS/PSD-95 PDZ dimer requires a preformed beta-finger structure from the nNOS PDZ domain. J. Mol. Biol. 303:359–370. [DOI] [PubMed] [Google Scholar]
- Venema, V.J., H. Ju, R. Zou, and R.C. Venema. 1997. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J. Biol. Chem. 272:28187–28190. [DOI] [PubMed] [Google Scholar]
- Wang, S., H. Yue, R.B. Derin, W.B. Guggino, and M. Li. 2000. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 103:169–179. [DOI] [PubMed] [Google Scholar]
- Xia, J., X. Zhang, J. Staudinger, and R.L. Huganir. 1999. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 22:179–187. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, J., M. Takahashi, H. Kanki, H. Yano-Yanagisawa, T. Tazunoki, E. Sawa, T. Nishitoba, M. Kamishohara, E. Kobayashi, S. Kataoka, and T. Sato. 1997. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J. Biol. Chem. 272:8539–8545. [DOI] [PubMed] [Google Scholar]
- Yang, B., D. Jung, J.A. Rafael, J.S. Chamberlain, and K.P. Campbell. 1995. Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J. Biol. Chem. 270:4975–4978. [DOI] [PubMed] [Google Scholar]
- Yokota, T., Y. Miyagoe, Y. Hosaka, K. Tsukita, S. Kameya, S. Shibuya, R. Matsuda, Y. Wakayama, and S. Takeda. 2000. Aquaporin-4 is absent at the sarcolemma and at perivascular astrocye endfeet in alpha1-syntrophin knockout mice. Proc. Japan Acad. 76:22–27. [Google Scholar]