Abstract
Many terrestrial plants respond to flooding with enhanced ethylene production. The roots of flooded plants produce 1-aminocyclopropane-1-carboxylic acid (ACC), which is transported from the root to the shoot, where it is converted to ethylene. In the roots, ACC is synthesized by ACC synthase, which is encoded by a multigene family. Previously, we identified two ACC synthase genes of tomato that are involved in flooding-induced ethylene production. Here, we report the cloning of LE-ACS7, a new tomato ACC synthase with a role early during flooding but also in the early wound response of leaves. The promoter of LE-ACS7 is tagged by a Sol3 transposon. A Sol3 transposon is also present in the tomato polygalacturonase promoter to which it conferred regulatory elements. Thus, Sol3 transposons may affect the regulation of LE-ACS7 and may be involved in the communication between the root and the shoot of waterlogged tomato plants.
Keywords: ethylene/gene expression/wounding/seedling hypocotyl/inverted repeat
Ethylene plays an important role in plant growth and development, including the control of germination, senescence, floral fading, and fruit ripening. Ethylene synthesis also is stimulated by stresses such as drought, wounding, and waterlogging (1, 2).
Ethylene is synthesized from S-adenosylmethionine via 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene (3). The reactions are catalyzed by ACC synthase (ACS) and ACC oxidase (ACO). Among the ethylene-inducing stresses, the phenomenon of waterlogging is a particularly interesting one. This is because the consequences of the ethylene produced in the shoot as a response to flooding in the root are well understood, because the system is a good example for the spatial separation of a biosynthetic pathway, and because it is the most convincing example of root-to-shoot communication involving a hormone (4).
Flooded tomato roots synthesize ACC, which is transported via xylem to the leaves within 6–12 h (5). In the leaves, ACC is converted by ACC oxidase to ethylene, which causes epinasty (6, 7). Leaves of transgenic tomatoes expressing antisense ACC oxidase produce less flooding-induced ethylene than untransformed controls (8, 9). The ethylene produced by waterlogged plants is interpreted as an early warning of deteriorating soil conditions, inducing changes above ground to increase stress tolerance (4). Flooding-induced ethylene is of agricultural importance, for instance in cotton, where it causes losses due to premature abscission of cotton bowls after waterlogging (10). For these reasons, it is important to understand the mechanism of flooding-induced ACC synthase activity in roots.
Hypoxia caused by flooding induces the production of ACC synthase mRNAs in roots (11–13). ACC synthase is encoded by a gene family, and in tomato seven members, LE-ACS1A, 1B, 2, 3, 4, 5, and 6 are known and differentially expressed (14–18).
Previously, we found that LE-ACS2, commonly known as the ACS gene that causes fruit ripening (14, 15, 19, 20), is induced in flooded roots (12). However, LE-ACS2 could not be responsible for the early flooding-induced ethylene production because ACC is delivered from root-to-shoot within 6 h after flooding (5, 7) but the LE-ACS2 transcript appears 8 h after flooding (12). LE-ACS3 also is induced in flooded roots, but half of its polyadenylated mRNA remains unspliced, a common phenomenon under anaerobiotic stress (12). The stress-induced splicing failures may reduce the amount of LE-ACS3 transcripts, leading to functional ACS protein. We searched for additional ACS transcripts, which contribute to the initial burst of ethylene produced by flooded plants.
Here, we report the cloning of a new tomato ACC synthase gene, LE-ACS7. Together with LE-ACS2, the LE-ACS7 transcript forms a rhythmic pattern that precedes the diurnal fluctuation of ethylene synthesis by leaves of flooded plants. We found a transposable element of the Sol3 family (21) in the promoter of LE-ACS7. The element is also present in the promoter of the tomato polygalacturonase (PG) gene to which it confers regulatory regions. Thus, Sol3 transposons may be involved in the communication of roots and shoots in waterlogged tomato plants.
MATERIALS AND METHODS
Plant Material.
Tomato plants (Lycopersicon esculentum Mill., cv. UC82B) were grown in 15-cm pots in a green house. Ten-week-old plants were used for the experiments. Fruits were harvested at the stages mature green, turning, pink, orange, and red. A suspension culture of cv. VFTN was maintained as described (18).
Induction of C2H4 Synthesis.
Young mature leaves were wounded with a brass wire brush, detached after various times, and divided into two parts. One part was assayed for C2H4 production and the second was frozen for RNA extraction. For wounding of stems and roots, the isolated tissues were cut into 5-mm long pieces and incubated on a moist filter paper for 6 h. For flooding, the roots were submerged in water for 0–260 h. Leaves and roots were collected and either frozen for RNA extraction or assayed for C2H4 production and ACC content. For C2H4 treatment, plants were incubated in sealed containers with 10 μl/liter C2H4 for 12 h, and the tissues then were frozen. For elicitor treatment, cell suspensions were incubated for 1 h in the presence of 200 μg/ml yeast elicitor. For auxin treatment, 5-mm sections from the top of the hypocotyl of 7-day-old etiolated seedlings were floated on 10 μM α-naphthaleneacetic acid for 90 min.
ACC and C2H4 Determination.
ACC was determined according to Lizada and Yang (22). Either the third youngest mature leaf or the root was used to determine C2H4 production with the procedure of Jackson and Campbell (23). The tissues were weighed and capped into a 50-ml flask, and after 30 min, a 1-ml gas sample was analyzed by gas chromatography.
Nucleic Acid Extraction and Reverse Transcriptase PCR and PCR.
Nucleic acids were extracted according to standard procedures (24). Total RNA (5 μg) was annealed with 1 μg of oligo-dT in 0.3 M NaCl, 3 mM EDTA, 10 mM Tris⋅HCl (pH 7.5), and 1 μl of RNAsin (Promega) at 50°C for 30 min. The mixture was adjusted to 1 mM each d-NTP, 1 mM DTT, 10 mM Tris⋅HCl (pH 8.4), 6 mM MgCl2, and 60 μg/ml actinomycin D in a final volume of 40 μl. Avian myeloblastosis virus reverse transcriptase (50 units) was added, and the mixture was incubated at 42°C for 2 h. The nucleic acids were precipitated and resuspended in 250 μl of diethyl-pyrocarbonate-treated water. PCR was carried out by using 5 μl of this solution. The primers used were (A) AYCCWTSWAATCCAYTRGGNAC and (B) ACWARNCCRAARCTNGACAT, corresponding to the conserved blocks 4 and 6 of ACC synthase (see ref. 25 for nomenclature). The primers used for amplification of genomic DNA were (C) CATGGCTAGCACAAAATCCAGA (corresponding to nt 2,685–2,707 of figure 3 of ref. 12) and (D) TACACTGATGTATGGGATAGAGTTCA (reverse complement of nt 3,425–3,451 of Fig. 1).
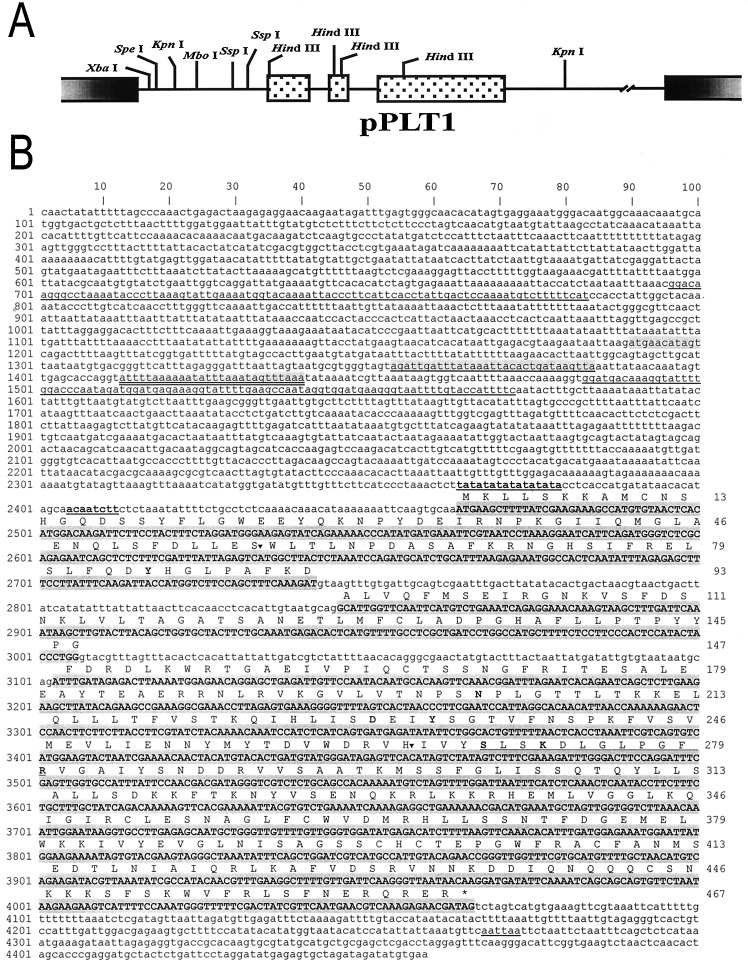
Figure 1.
Organization of and the complete nucleotide sequence of LE-ACS7. (A) The graph shows the organization of the λ-EMBL3 clone pPLT1. Three exons of LE-ACS7 are represented by dotted boxes, and two introns are represented by lines connecting the three exons. (B) Complete nucleotide sequence of the LE-ACS7 gene. The coding sequence is capitalized and highlighted, and introns and untranslated regions are indicated in lowercase. Putative tata-box, cap site, and polyadenylation sites are underlined in bold. A long, imperfect, inverted repeat is underlined. Regions corresponding to regions C, D, and E of the tomato polygalacturonase promoter (31) are shaded, shaded and underlined, and shaded and double underlined, respectively. The amino acid sequence is presented above the DNA sequence and numbered separately. The dodecapeptide that is part of the reactive center of ACC synthase is underlined. The star identifies the stop codon. The seven invariant residues that bind the pyridoxal phosphate coenzyme in various aspartate aminotransferases are conserved across all aspartate aminotransferases, and ACC synthase are in bold. The region of the p4ROM2 probe is delimited by two inverted triangles (nt 2,632–3,456).
Ribonuclease Protection (RPA) Assay.
The plasmids used for RPA assay were pBTAS1, pBTAS2 (17), and pCS14HP (corresponding to nt 3,202–3,494 of Fig. 1), corresponding to the tomato ACC synthase genes LE-ACS2, LE-ACS3, and LE-ACS7, respectively. The plasmids were linearized, and radioactive RNA probes were prepared by in vitro transcription with T7 RNA polymerase at 37°C for 1 h in the presence of 70 μCi of 32P-UTP. Probes were purified in a 5% acrylamide/8 M urea gel. The assay was carried out with a High-Speed hybridization RPA assay kit from Ambion (Austin, TX). Fragments were separated in 5% acrylamide/8 M urea gels, and the gels were autoradiographed.
Genomic Cloning and DNA Sequencing.
Tomato genomic libraries were from CLONTECH or were provided by Charles S. Gasser (26). Plating, plaque lifting, and filter hybridization were conducted according to standard procedures (27). The purified 800-bp EcoRI insert of p4ROM2 was labeled with [α-32P]-dATP and was used for screening. Positively hybridizing clones were purified and digested with HindIII, and the fragments were subcloned into pBSII SK+. The subclones were sequenced in both directions by using Sequenase 2.0 and 35S-dATP.
RESULTS
Isolation, Cloning, and Characterization of LE-ACS7.
Twelve first-strand cDNA libraries, prepared from intact, wounded, and C2H4-treated roots, stems, leaves, auxin-treated hypocotyls, elicitor-treated cell suspensions, and fruit pericarp at five different ripening stages were combined and amplified with primers A and B. To remove known ACS transcripts, the PCR product was digested with AvaI, BclI, ClaI, HinfI, and StyI, TA-cloned, and sequenced. One clone, p4ROM1 (320 nt), represented an ACC synthase, designated LE-ACS7. Because p4ROM1 was 80% identical to LE-ACS3, a more divergent probe was isolated for library screening. This probe was obtained by amplification of genomic DNA with primers D, specific for LE-ACS7, and C, experimentally selected from a number of forward primers previously used for sequencing LE-ACS3 and cross annealing to LE-ACS7. The resulting 818-nt PCR clone, p4ROM2, was less similar to LE-ACS3 and had two introns very divergent from LE-ACS3. Genomic libraries were screened with p4ROM2, and positively hybridizing clones were selected after four screens, resulting in the λ-EMBL3 clone pPLT1, which contains the complete LE-ACS7 gene (Fig. 1A).
The LE-ACS7 Gene.
LE-ACS7 encodes a protein of 467 aa, a molecular mass of 53.1 kDa, and a pI of 8.3 (Fig. 1B). The active site center dodecapeptide sequence is identical to the minor of two peptides isolated earlier from the tomato fruit (28). LE-ACS7 is interrupted by two introns and is highly homologous to LE-ACS3. The gene represents a class III ACC synthase, the class with the highest pI (Fig. 2). LE-ACS7 is very similar to the potato ST-ACS2 (29). LE-ACS7 was mapped to the top of tomato chromosome 2, between the markers TG31 and CT106A, not coinciding with the location of any known tomato mutant phenotypes (30).
Figure 2.
Phylogenetic relationship of the tomato ACC synthase isoforms. The potato ST-ACS2 (29) also is included to demonstrate its high degree of similarity to LE-ACS7. DNA sequences encoding the polypeptide between blocks 4 and 6 (25) were used to construct the tree.
LE-ACS7 Is Induced Early After Flooding.
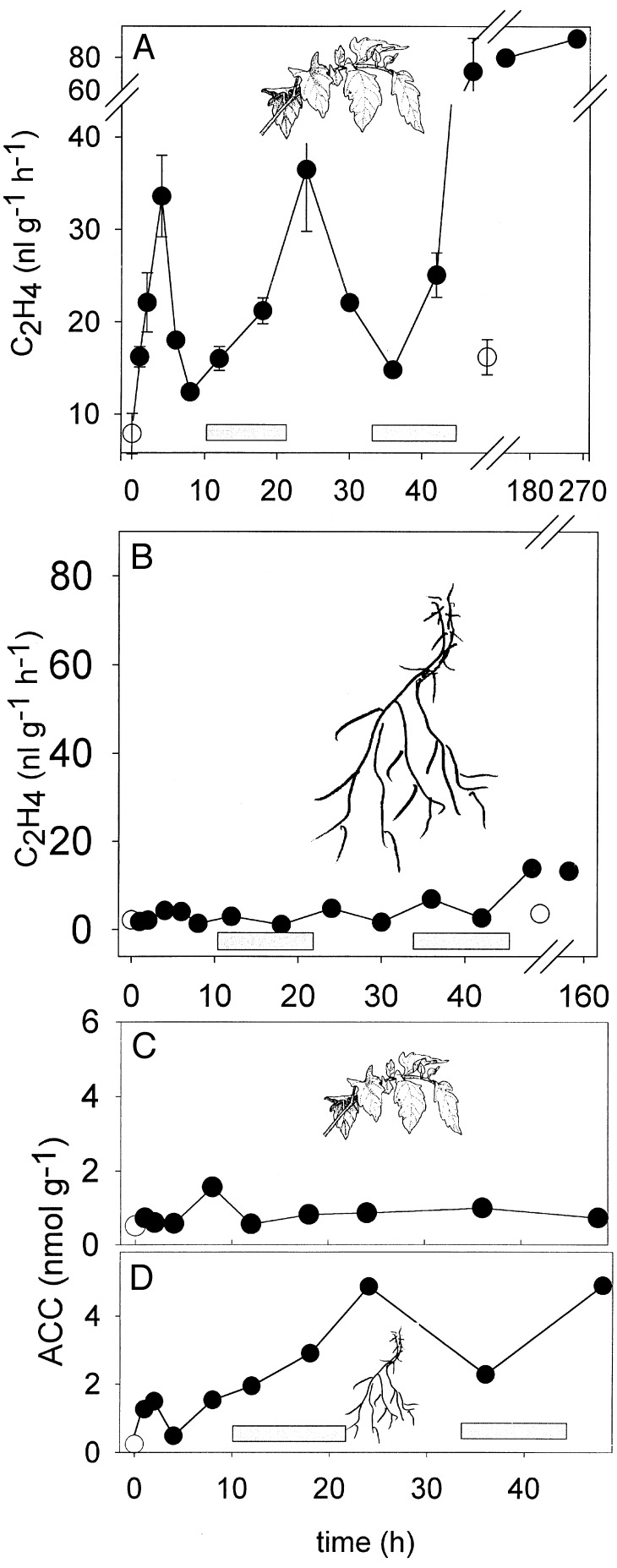
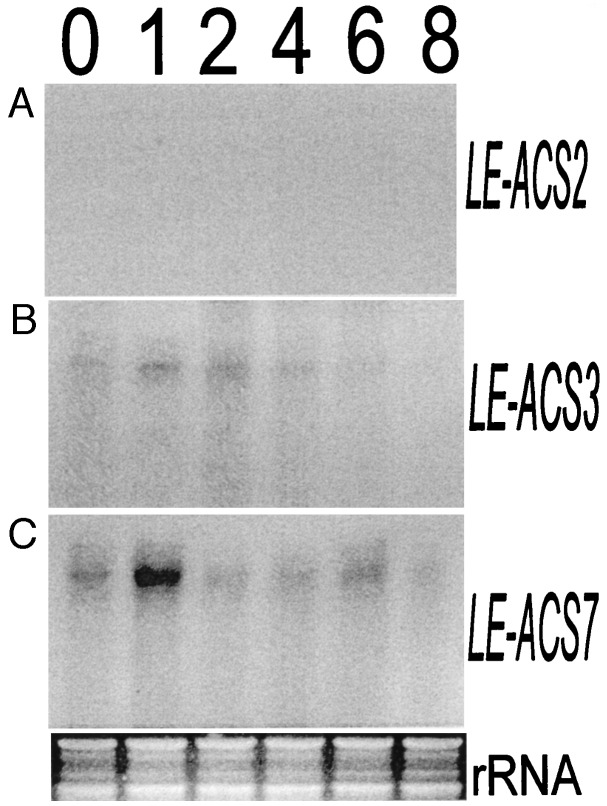
Preliminary experiments showed LE-ACS7 is expressed in roots, and we investigated its role in flooding. Flooding treatments started at 8 a.m., and after 1 h, the leaves exhibited a steep increase of C2H4 production that peaked during mid-day. (Fig. 3A). Subsequently, the rate of C2H4 synthesis dropped and fluctuated in a similar rhythm with second and third maxima after 24 and 48 h. These peaks coincided with the light periods. After 5 and 10 days of flooding, and at daytime, C2H4 synthesis remained high. In contrast, the C2H4 released by roots remained at a basal level during the first 2 days of flooding (Fig. 3B). After that, when the plants showed severe symptoms, the rate of C2H4 production increased but remained always at <1/10 of the C2H4 biosynthetic rate of leaves. Apart from an initial increase, the ACC content in leaves remained low (Fig. 3C). The ACC content of flooded roots was higher than in leaves and fluctuated. Clear morphological alterations were noted such as epinasty and the formation of aerial roots on lower stems and, after prolonged flooding, yellowing and senescence of leaves. Besides LE-ACS7, we studied LE-ACS2 and LE-ACS3, but not LE-ACS4 and LE-ACS5, which are not induced in flooded roots (12). The transcripts of LE-ACS2, LE-ACS3, and LE-ACS7 were not present in leaves of flooded plants, the tissues with the highest rate of C2H4 synthesis (Fig. 4 A–C). However, they were found in flooded roots. LE-ACS2 was induced 8 h after flooding (Fig. 4D). Of interest, this transcript fluctuated, became reduced after 12 h, and increased again after 24 h. The peaks of maximal LE-ACS2 transcript abundance in roots coincided with the nights when the C2H4 production of the leaves was low. A minor amount of LE-ACS3 was detectable in unflooded roots, and flooding strongly induced that transcript (Fig. 4E). The abundance of LE-ACS3 remained high at all times. Like LE-ACS3, there was a basal level of LE-ACS7 in unflooded roots (Fig. 4F). LE-ACS7 was induced rapidly at the early stages of flooding, preceding the initial peak of flooding-induced C2H4 synthesis in the leaves, but it became less abundant after 12 h. The absence of these transcripts in leaves of flooded plants led to the question whether leaves cannot express them at all or if they express them when challenged by a different stress. In several wounding experiments, C2H4 production peaked 2 h after wounding and then decreased (data not shown). LE-ACS2, known to be wound-inducible in the fruit, was not detectable in wounded leaves (Fig. 5A), and there was only a minor amount of LE-ACS3 (Fig. 5B). However, there was a transient increase of LE-ACS7 mRNA early after the wounding of leaves, suggesting that this gene is involved in the formation of wound ethylene by vegetative tissues and with an expression pattern resembling the one of LE-ACS7 in flooded roots (Fig. 5C).
Figure 3.
Ethylene production and ACC content of flooded tomato plants. (A) Ethylene produced by leaves. A break was inserted. Data from two independent experiments were pooled. [Error bars represent SEM (n ≥ 3).] (B) Ethylene produced by roots. (C) ACC content of leaves. The mean of two independent experiments is plotted. (D) ACC content of roots. The mean of two independent experiments is plotted. ○, tissues from unflooded control plants; •, tissues from flooded plants. The dark periods in the green house are symbolized by the shaded bars.
Figure 4.
Expression of ACC synthase genes LE-ACS2, LE-ACS3, and LE-ACS7 in leaves and roots of flooded plants. RNA was extracted at various times after flooding, and 100 μg for each time point and gene was analyzed by RPA assay. A section of an ethidium bromide-stained agarose gel with ribosomal RNA bands is depicted under F to show that even amounts of RNA were used for the assays. The times of flooding (h) are given above the figures. (A–C) Expression of LE-ACS2, LE-ACS3, and LE-ACS7, respectively, in leaves and (D–F) in roots.
Figure 5.
Expression of LE-ACS2, LE-ACS3, and LE-ACS7 in wounded leaves. The times after wounding (h) are shown above the figure. For each time point and gene, 100 μg of RNA was analyzed by RPA assay. (A) LE-ACS2. (B) LE-ACS3. (C) LE-ACS7.
The Promoter of LE-ACS7 Is Tagged by a Sol3 Element.
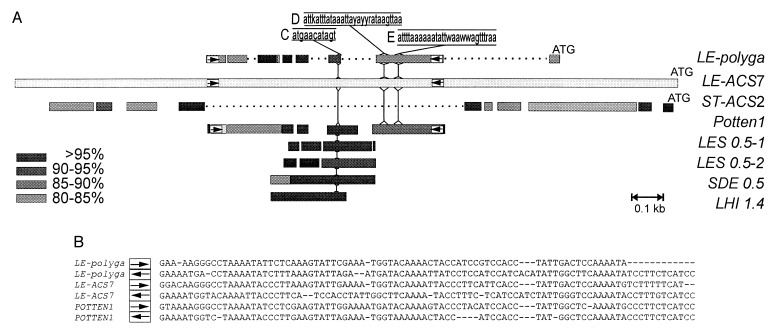
The promoter of LE-ACS7 revealed an exceptionally high degree of similarity over >1 kb to the promoter of the wound-repressed potato ACC synthase ST-ACS2 (Fig. 6A) (29). However, the LE-ACS7 promoter contains an insertion of 950 bp not present in ST-ACS2. This insertion is very similar to a region in the promoter of the tomato polygalacturonase (PG) gene that has been shown by deletion analysis to contain positive regulatory regions and, by DNaseI footprinting, the nuclear protein-binding regions designated C, D, and E (Fig. 6A) (31). This 852-nt long region of the PG promoter subsequently was shown to be a transposon of the Sol3 type (21), a solanaceous transposon family that occurs in tomato and potato but not in tobacco (32). The LE-ACS7 promoter contains domains similar to the regions C, D, and E of the PG promoter (Figs. 1 and 6A). Several Sol3 elements occurring in wild forms of the potato and tomato are highly similar to the insert in the LE-ACS7 promoter and also contain domains corresponding to C, D, and E. The long terminal inverted repeats that define Sol3 elements also were found in LE-ACS7 and are flanking the Sol3 region (Figs. 1 and 6B). The element in the LE-ACS7 promoter was designated LEACS7Sol3.
Figure 6.
Comparison of the LE-ACS7 promoter with other plant promoters and with Sol3 elements. (A) The bars represent alignments of DNA sequences as revealed by a gapped blast search (44). The sequence names are printed on the right of each bar, and the distance scale (0.1 kb) is shown. The promoter of LE-ACS7 is depicted as a light grey bar, beginning on the left with nt 1 and ending on the right with the initiation codon ATG, i.e., nt 2,466 of Fig. 1. Blocks of DNA sequence identity to the LE-ACS7 promoter are aligned above and below the LE-ACS7 promoter, at various grey shades to represent their percentage of identity to the corresponding regions of the LE-ACS7 promoter, as indicated in the legend. Blocks with identities of <80% to the LE-ACS7 promoter were not plotted. Gaps (… .) were inserted in the promoters of ST-ACS2 and LE-polyga. White boxes with arrows indicate the positions of the long terminal inverted repeats characteristic of Sol3 transposons. The position of the nuclear protein binding DNA sequences C, D, and E in the positive regulatory region of the PG promoter (nucleotides −412 to −806) (31) and their consensus sequence with LE-ACS7 are shown. Vertical lines show where these regions are located on the LE-ACS7 promoter and on various Sol3 elements. The Sol3 DNA sequences used are: Solanum tuberosum (Potten1) GenBank accession no. U91987; Solanum demissum (SDE 0.5) accession no. U91992; Lycopersicon esculentum (LES 0.5–1), accession no. U91989; (LES 0.5–2), accession no. U91990; Lycopersicum hirsutum (Lhi 1.4), accession no. U91988. (B) Alignment of the long terminal inverted repeats of LE-polyga, LE-ACS7, and Potten1.
DISCUSSION
We report the cloning of a tomato ACC synthase gene, LE-ACS7, involved early in the production of flooding-induced ethylene, and a tomato ACC synthase gene shown to be induced after wounding of vegetative tomato tissues. Wound-induced LE-ACS7 transcript accumulation was transient and preceded the production of wound ethylene. The closely related ACC synthase, ST-ACS2, is wound repressed transiently (29) and lacks the Sol3 element of LE-ACS7. LE-ACS7 is induced early after flooding, suggesting that it is a primary gene in the root-to-shoot communication of the flooded plant.
Flooding-induced ethylene produced by leaves fluctuated and peaked during daytime when transpiration is high, as previously observed (33). If ACC synthesis in roots remained constant after flooding, one would expect that ACC in the roots accumulates at night, when transpiration is low. However, the ACC content of roots was high during the day and dropped at night, indicating that ACC synthase activity in roots, once induced, fluctuates in a diurnal rhythm. One possible mechanism for that would be via sequential transcriptional activation of ACC synthase genes (34). Time-controlled synthesis of flooding-induced ACC would conserve nightly resources of the stressed plant yet provide sufficient substrate during daytime for the LE-ACO1 gene, which is induced in the shoots after flooding of the roots (7). Many unflooded plants, including the tomato, show natural rhythms of ethylene evolution with peaks during daylight, and the Sl-ACO1 transcript in leaves from Stellaria longipes fluctuates accordingly (35). Fluctuation of ACC production of flooded roots may have evolved to most efficiently fit in and exploit the preexisting natural rhythm of the healthy plant to produce flooding ethylene during daytime. During daytime, it might be most needed to reduce light interception via epinasty to maintain the water balance of the flooded plants (6).
We previously cloned the gene LE-ACS3 and showed that it is induced early after flooding and that its promoter contains anaerobiosis response elements (12). Approximately half of the LE-ACS3 transcript remained unspliced and might not lead to a functional enzyme. LE-ACS4 and LE-ACS5 were not induced in flooded tomato roots. However, LE-ACS2 was induced 10 h after flooding in the variety studied (cv. VFN8) (12). The present study, using cv. UC82B, confirms this result and suggests that signaling for induction of LE-ACS2 in flooded roots is identical in these varieties. The initial burst of flooding-induced ethylene precedes the induction of LE-ACS2, and, hence, other ACC synthase genes must be responsible for this initial burst. After induction, the abundance of LE-ACS2 mRNA fluctuated, peaked at night, and preceded the daily waves of ACC production in roots and ethylene evolution from shoots. LE-ACS7 was induced early and transiently and could, in combination with LE-ACS2, well account for the sustained waves of ethylene production as shown in Fig. 3A.
There are several explanations for the differential induction of these ACC synthase transcripts. For instance, they may be expressed in different root tissues such as the cortex and the central cylinder. The signaling pathway for LE-ACS7 and LE-ACS3 may be identical but differ from the one for late induction of LE-ACS2. Kinetically, it appears that LE-ACS3 and LE-ACS7 directly sense hypoxia in the roots whereas the delayed and fluctuating induction of LE-ACS2 may be due to a secondary stimulus. Since LE-ACS2 was more abundant during dark periods when transpiration is low, this stimulus might be a compound that accumulates in the roots at night. Alternatively, slight increases of the internal ethylene concentration in roots, beyond our limit of detection, might be that stimulus. LE-ACS2 is ethylene-inducible in the fruit and is responsible for autocatalytic ethylene production (15, 20). The ethylene receptor Nr also is induced early during fruit ripening, preceding the induction of the ethylene-inducible E8 gene and preventing ripening when mutated (36). Nr is ethylene-inducible in tomato fruits once they have proceeded from the immature green to the mature green stage. The small amounts of ethylene produced by the immature fruit may be involved in that transition (37). The signal for induction of LE-ACS2 and the onset of autocatalytic ethylene production in the fruit may be transduced via the Nr protein, once the fruit tissue has achieved the developmental competence. Of interest, flooding also induces the Nr-like ethylene receptor (38). Taken together, it may be hypothesized that an analogous mechanism is operating in the flooded root, using small amounts of ethylene produced by early flooding-induced ACC synthases such as LE-ACS7 to induce Nr and leading to the late induction of LE-ACS2 as observed earlier (12). A similar mechanism also might cause induction of LE-ACO1 (7) in leaves of flooded plants. We are not aware of studies dealing with the effect of flooding on the Nr mutant of tomato.
We are unable to assess the relative contribution of the two early ACC synthase genes, LE-ACS3 and LE-ACS7, to the initial burst of ethylene, but the present data show that LE-ACS7 plays a role early during flooding. The induction pattern of LE-ACS7 by flooding and wounding is almost identical, suggesting that this gene has an early and transient function during stress. No previous reports described a wound-inducible ACC synthase tomato gene that could account for the short and transient burst of ethylene produced by wounded leaves.
LE-ACS7 is related closely to ST-ACS2, and their similarity also is found in the promoters. The coding regions of both genes are only interrupted by two introns, in contrast to the majority of the ACC synthases with three introns. The sequence identity of these two promoters is unusual and is not found between any other ACS promoters, not even the duplicated ACS genes CP-ACS1A/CP-ACS1B or ST-ACS1A/ST-ACS1B. It is likely that ST-ACS2 and LE-ACS7 evolved from a common ancestor gene and that there are additional close relatives in the Solanaceae. The only ACC synthase promoter cloned from a solanaceous plant other than potato and tomato (PH-ACS1; GenBank accession no. PHU64804) does not resemble LE-ACS7 or ST-ACS2. Chloroplast DNA evidence shows that potato and tomato are close relatives, whereas tobacco is quite distant (39). Tobacco does not contain Sol3 elements (32). Thus, the Sol3 transposon excised from the common ancestor of ST-ACS2 and LE-ACS7 or inserted into the LE-ACS7 promoter only recently. We confirmed by PCR the presence of LEACS7Sol3 in both the LE-ACS7 promoters from cv. VF36 (used for preparation of the genomic library) and cv. UC82B (used for the flooding and wounding experiments). However, more work is needed to investigate when the transposition occurred and if and how it affected the flooding and wound response of tomato plants. The Sol3 element in the tomato PG promoter contributes positive regulatory regions for PG expression (31), and in a number of cases transposition into the promoter altered the plant phenotype (31, 40, 41).
We support the view that ACC synthase gene families should not be seen as a mere case of gene redundancy but as a case of overlapping gene functions (42). The ACC synthase gene LE-ACS7 is part of that family of overlapping functions and plays a role early in the production of stress-ethylene. Recently, transposon-like elements were discovered in the tomato ACC oxidase promoters LE-ACO1 and LE-ACO3 (43). Together with the evidence presented here, it may be hypothesized that transposable elements have increased the flexibility of the tomato ethylene biosynthetic gene apparatus, and this may play a role in the root-to-shoot communication of waterlogged plants.
Acknowledgments
We thank Ms. Theresa Fulton and Ms. Candi Lewis from the laboratory of Dr. Steven D. Tanksley for restriction fragment length polymorphism mapping the LE-ACS7 gene. We thank Dr. Charles S. Gasser for his tomato genomic libraries and Drs. Richard G. Olmstead, Roger Chetelat, Charles M. Rick, and David Spooner for useful discussions on the phylogeny of the Solanaceae. We are grateful to Dr. William Belknap and Prof. Don Grierson for reviewing the manuscript and for helpful discussion. This research was supported by the National Science Foundation Grant MCB-9303801, the Treubelfonds of Freiwillige Akademische Gesellschaft, Basel, Switzerland, and the Hong Kong Research Grant Council (HKU 390/94M). O.Y.S. was supported by a Ph.D. studentship of the University of Hong Kong and a Swire Scholarship from John Swire & Sons (H.K.) Ltd.
ABBREVIATIONS
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACS
ACC synthase
- ACO
ACC oxidase
- RPA
ribonuclease protection assay
- PG
polygalacturonase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF043122).
References
- 1.Jackson M B. Annu Rev Plant Physiol. 1985;36:145–175. [Google Scholar]
- 2.Abeles F B, Morgan P W, Saltveit M E. Ethylene in Plant Biology. San Diego: Academic; 1994. [Google Scholar]
- 3.Adams D O, Yang S F. Proc Natl Acad Sci USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson M B. Trends Plant Sci. 1997;2:22–28. [Google Scholar]
- 5.Bradford K J, Yang S F. Plant Physiol. 1980;65:322–326. doi: 10.1104/pp.65.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford K J, Hsiao T C, Yang S F. Plant Physiol. 1982;70:1503–1507. doi: 10.1104/pp.70.5.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English P J, Lycett G W, Roberts J A, Jackson M B. Plant Physiol. 1995;109:1435–1440. doi: 10.1104/pp.109.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton A J, Lycett G W, Grierson D. Nature (London) 1990;346:284–287. [Google Scholar]
- 9.English P J, Lycett G W, Roberts J A, Hall K C, Jackson M B. J Exp Bot. 1995;45:33. [Google Scholar]
- 10.Guinn G. Plant Physiol. 1982;69:349–352. doi: 10.1104/pp.69.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarembinski T I, Theologis A. Mol Biol Cell. 1993;4:363–373. doi: 10.1091/mbc.4.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson D C, Oetiker J H, Yang S F. J Biol Chem. 1995;270:14056–14061. doi: 10.1074/jbc.270.23.14056. [DOI] [PubMed] [Google Scholar]
- 13.Zarembinski T I, Theologis A. Plant Mol Biol. 1997;33:71–77. doi: 10.1023/b:plan.0000009693.26740.c3. [DOI] [PubMed] [Google Scholar]
- 14.Van der Straeten D, Van Wiemeersch L, Goodman H M, Van Montagu M. Proc Natl Acad Sci USA. 1990;87:4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottmann W H, Peter G F, Oeller P W, Keller J A, Shen N F, Nagy B P, Taylor L P, Campbell A D, Theologis A. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- 16.Olson D C, White J A, Edelman L, Harkins R N, Kende H. Proc Natl Acad Sci USA. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip W-K, Moore T, Yang S F. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oetiker J H, Olson D C, Shiu O Y, Yang S F. Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- 19.Parsons B L, Mattoo A K. Plant Mol Biol. 1991;17:453–464. doi: 10.1007/BF00040639. [DOI] [PubMed] [Google Scholar]
- 20.Oeller P W, Min-Wong L, Taylor L P, Pike D A, Theologis A. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- 21.Oosumi T, Garlick B, Belknap W R. Proc Natl Acad Sci USA. 1995;92:8886–8890. doi: 10.1073/pnas.92.19.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizada M C, Yang S F. Anal Biochem. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- 23.Jackson M B, Campbell J D. Planta. 1976;129:273–274. doi: 10.1007/BF00398271. [DOI] [PubMed] [Google Scholar]
- 24.Katharina P, Reinhard K, de Vries S, Ton B. In: Plant Molecular Biology Manual. Gelvin S B, Schilperoort R A, editors. Dordrecht, the Netherlands: Kluwer; 1995. pp. 1–4. [Google Scholar]
- 25.Dong J G, Kim W T, Yip W K, Thompson G A, Li L, Bennett A B, Yang S F. Planta. 1991;185:38–45. doi: 10.1007/BF00194512. [DOI] [PubMed] [Google Scholar]
- 26.Budelier K A, Smith A G, Gasser C A. Mol Gen Genet. 1990;224:183–192. doi: 10.1007/BF00271551. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Yip W K, Dong J G, Kenny J W, Thompson G A, Yang S F. Proc Natl Acad Sci USA. 1990;87:7930–7934. doi: 10.1073/pnas.87.20.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Destefano-Beltran L J, Van Caeneghem W, Gielen J, Richard L, Van Montagu M, Van der Straeten D. Mol Gen Genet. 1995;246:496–508. doi: 10.1007/BF00290453. [DOI] [PubMed] [Google Scholar]
- 30.Pillen K, Omaira P, Lewis C B, Tanksley S D. In: Genome Mapping in Plants. Paterson A, editor. Austin, TX: R. G. Landes Co.; 1996. pp. 281–308. [Google Scholar]
- 31.Montgomery J, Pollard V, Deikman J, Fischer R L. Plant Cell. 1993;5:1049–1062. doi: 10.1105/tpc.5.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oosumi T, Belknap W R. J Mol Evol. 1997;45:137–144. doi: 10.1007/pl00006213. [DOI] [PubMed] [Google Scholar]
- 33.Jackson M B, Gales K, Campbell J D. J Exp Bot. 1978;29:183–193. [Google Scholar]
- 34.Schlagnhaufer C D, Arteca R N, Pell E J. Plant Mol Biol. 1997;35:683–688. doi: 10.1023/a:1005857717196. [DOI] [PubMed] [Google Scholar]
- 35.Kathiresan A, Reid D M, Chinnappa C C. Planta. 1996;199:329–335. doi: 10.1007/BF00195723. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson J Q, Lanahan M B, Yen H-C, Giovannoni J J, Klee H J. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- 37.McMurchie E J, McGlasson W B, Eaks I L. Nature (London) 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- 38.Vriezen W H, Van Rijn C P E, Voesenek L A C J, Mariani C. Plant J. 1997;11:1265–1271. doi: 10.1046/j.1365-313x.1997.11061265.x. [DOI] [PubMed] [Google Scholar]
- 39.Olmstead R G, Sweere J A, Spangler R E, Bohs L, Palmer J D. In: Phylogeny and Provisional Classification of the Solanaceae Based on Chloroplast DNA. Nee M, Symon D, editors. 1998. inpress. [Google Scholar]
- 40.Coen E S, Carpenter R, Martin C. Cell. 1986;47:285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- 41.Weil C F, Wessler S R. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:527–552. [Google Scholar]
- 42.Mulligan R M, Chory J, Ecker J R. Proc Natl Acad Sci USA. 1997;94:2793–2795. doi: 10.1073/pnas.94.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blume B, Barry C S, Hamilton A J, Bouzayen M, Grierson D. Mol Gen Genet. 1997;254:297–303. doi: 10.1007/s004380050419. [DOI] [PubMed] [Google Scholar]
- 44.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]