Abstract
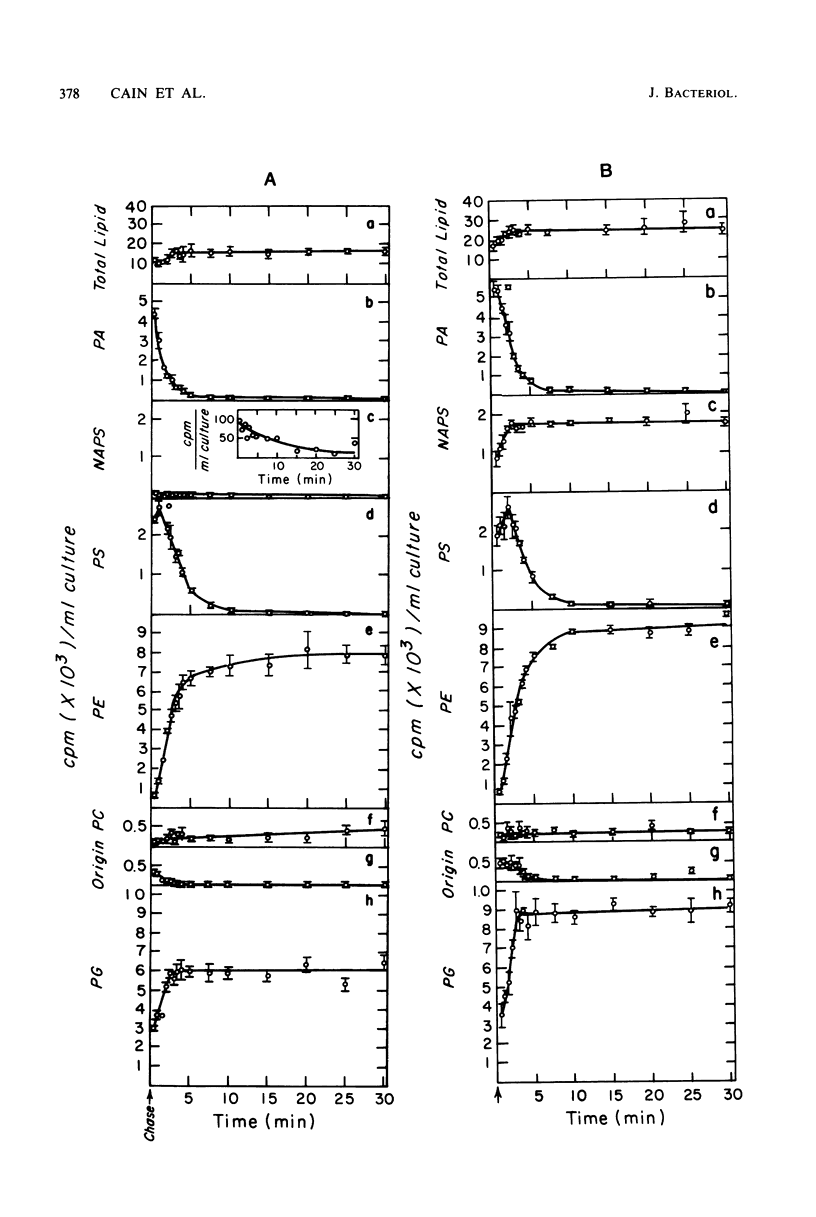
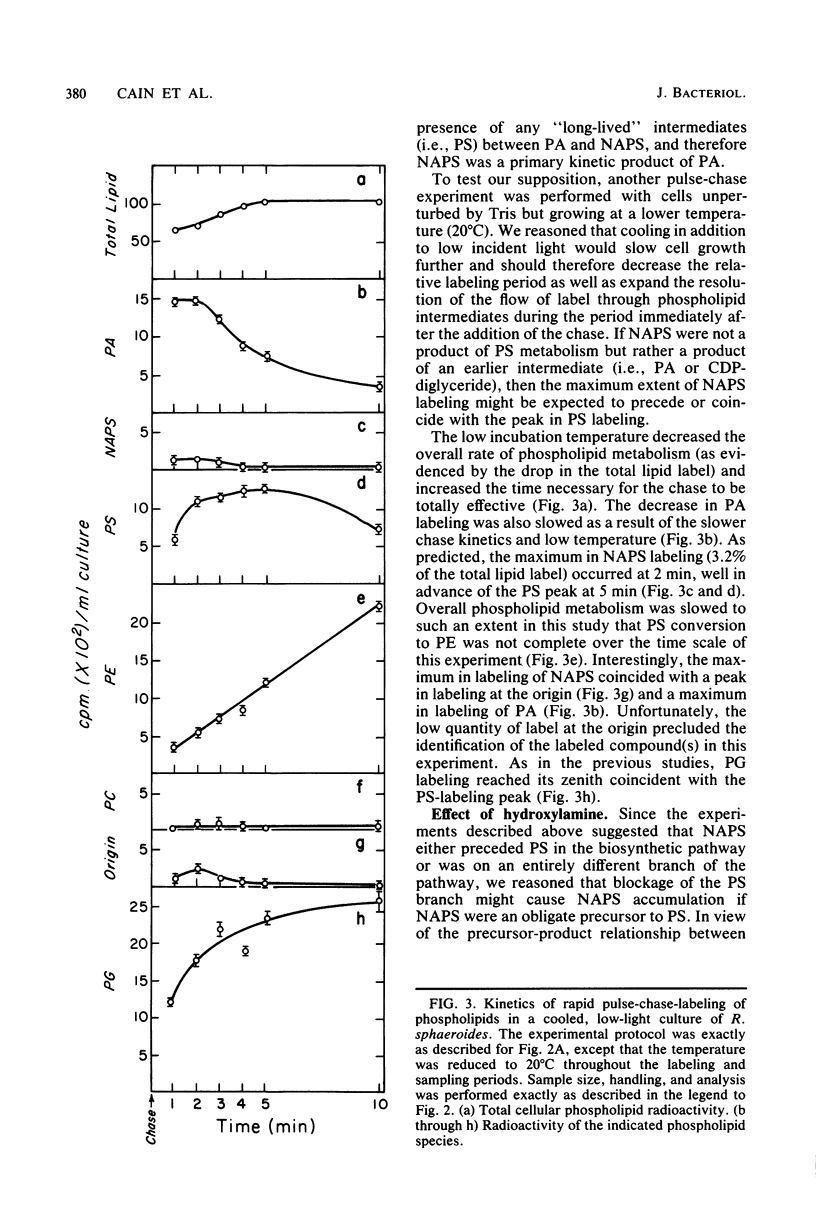
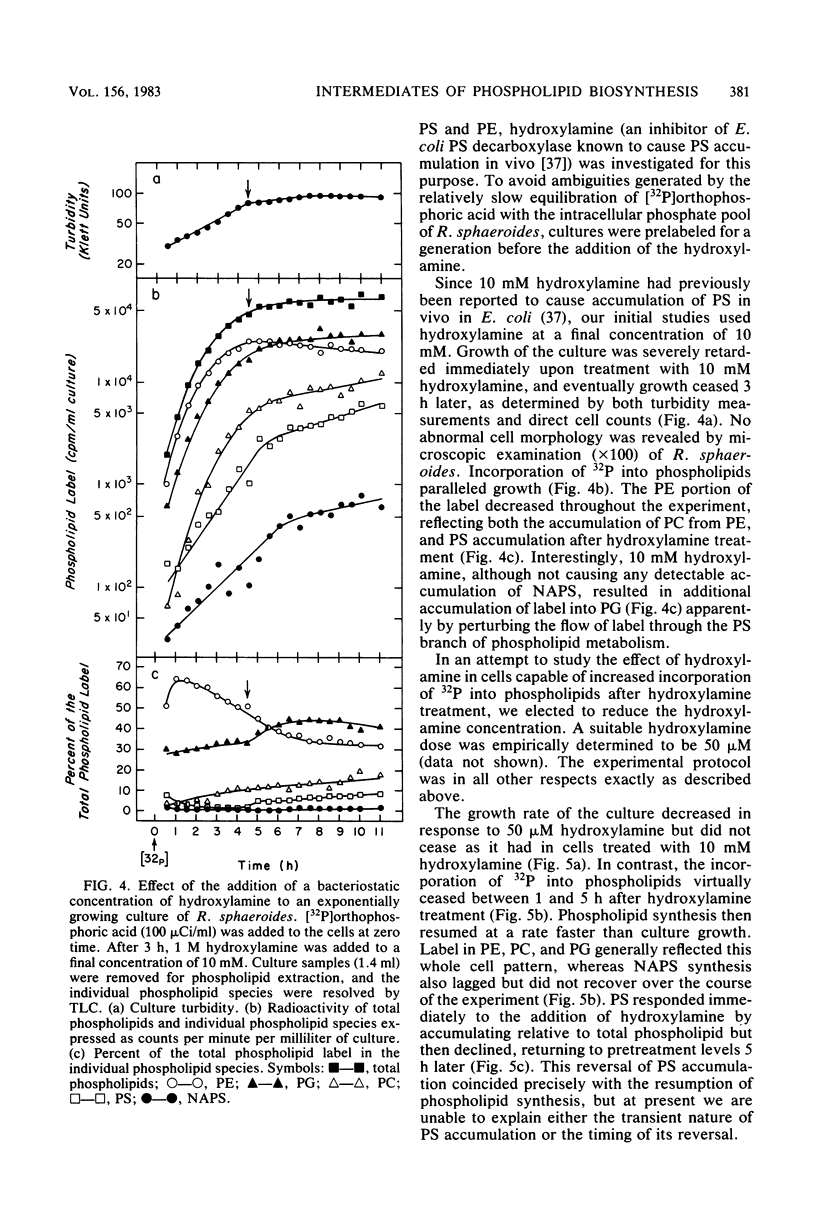
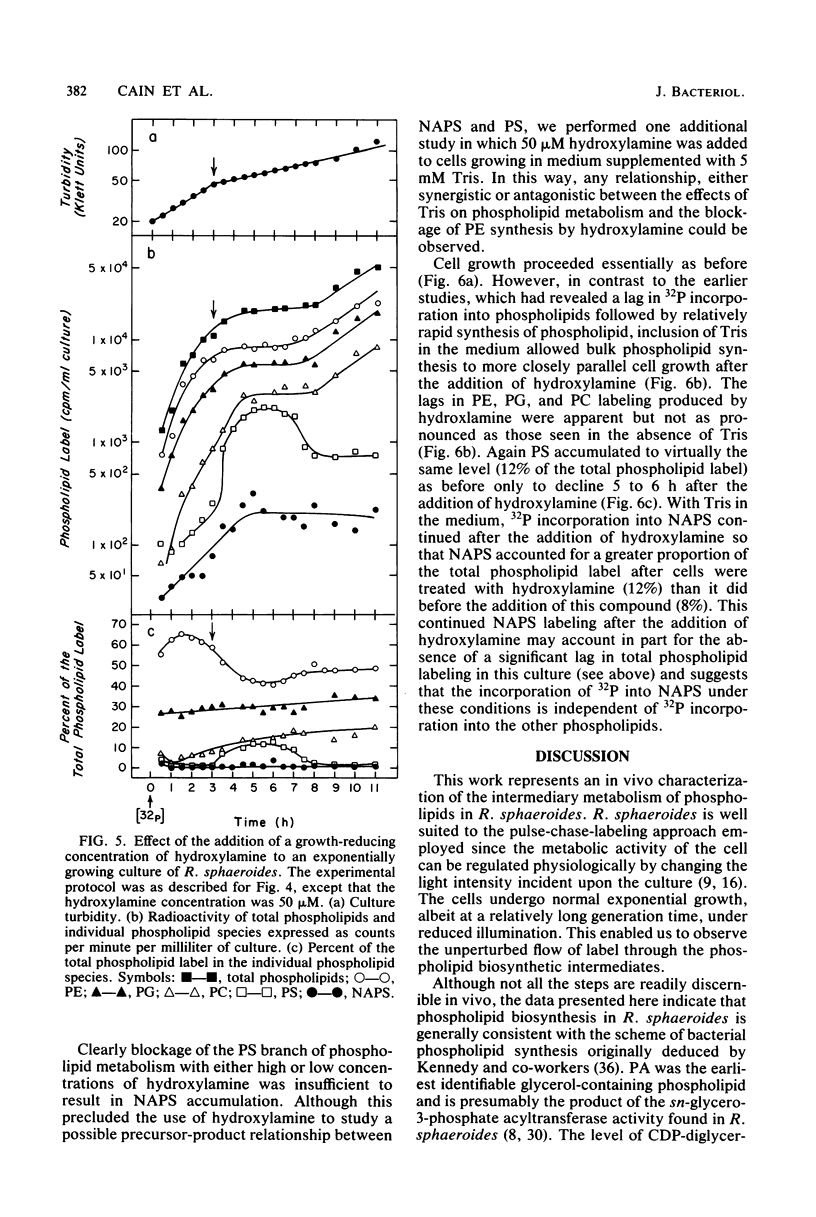
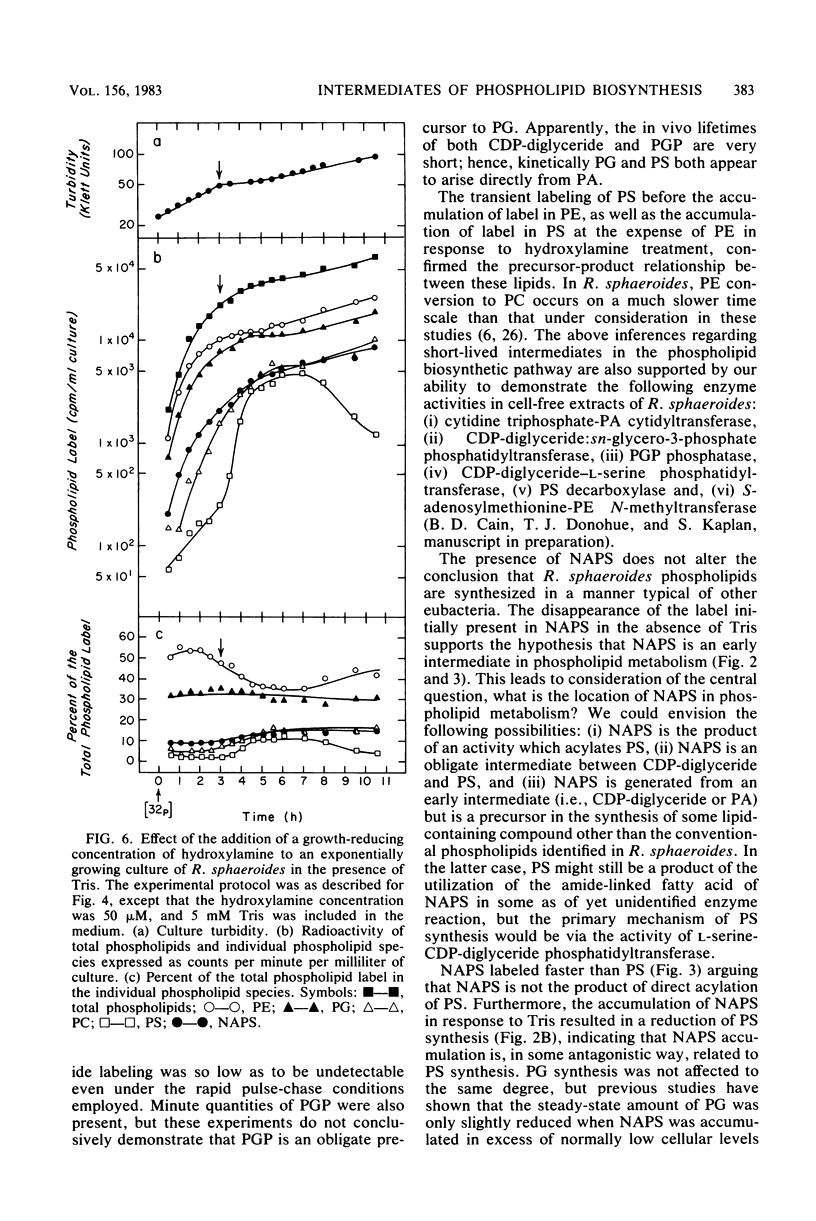
The in vivo metabolic pathways of phospholipid biosynthesis in Rhodopseudomonas sphaeroides have been investigated. Rapid pulse-chase-labeling studies indicated that phosphatidylethanolamine and phosphatidylglycerol were synthesized as in other eubacteria. The labeling pattern observed for N-acylphosphatidylserine (NAPS) was inconsistent with the synthesis of this phospholipid occurring by direct acylation of phosphatidylserine (PS). Rather, NAPS appeared to be kinetically derived from an earlier intermediate such as phosphatidic acid or more likely CDP-diglyceride. Tris-induced NAPS accumulation specifically reduced the synthesis of PS. Treatment of cells with a bacteriostatic concentration of hydroxylamine (10 mM) greatly reduced total cellular phospholipid synthesis, resulted in accumulation of PS, and stimulated the phosphatidylglycerol branch of phospholipid metabolism relative to the PS branch of the pathway. When the cells were treated with a lower hydroxylamine dosage (50 microM), total phospholipid synthesis lagged as PS accumulated, however, phospholipid synthesis resumed coincident with a reversal of PS accumulation. Hydroxylamine alone was not sufficient to promote NAPS accumulation but this compound allowed continued NAPS accumulation when cells were grown in medium containing Tris. The significance of these observations is discussed in terms of NAPS biosynthesis being representative of a previously undescribed branch of the phospholipid biosynthetic sequence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Al-Bayatti K. K., Takemoto J. Y. Phospholipid topography of the photosynthetic membrane of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Sep 15;20(19):5489–5495. doi: 10.1021/bi00522a022. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baumgardner D., Deal C., Kaplan S. Protein composition of Rhodopseudomonas sphaeroides outer membrane. J Bacteriol. 1980 Jul;143(1):265–273. doi: 10.1128/jb.143.1.265-273.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cain B. D., Deal C. D., Fraley R. T., Kaplan S. In vivo intermembrane transfer of phospholipids in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1981 Mar;145(3):1154–1166. doi: 10.1128/jb.145.3.1154-1166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain B. D., Donohue T. J., Kaplan S. Kinetic analysis of N-acylphosphatidylserine accumulation and implications for membrane assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Nov;152(2):607–615. doi: 10.1128/jb.152.2.607-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T. B., Lueking D. R. Long-chain fatty acid assimilation By rhodopseudomonas sphaeroides. J Bacteriol. 1983 Feb;153(2):782–790. doi: 10.1128/jb.153.2.782-790.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Kaplan S. Light-dependent regulation of the synthesis of soluble and intracytoplasmic membrane proteins of Rhodopseudomonas sphaeroides. J Bacteriol. 1983 Jan;153(1):465–474. doi: 10.1128/jb.153.1.465-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Lueking D. R., Kaplan S. Intermembrane phospholipid transfer mediated by cell-free extracts of Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Feb 10;254(3):721–728. [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Alterations in the phospholipid composition of Rhodopseudomonas sphaeroides and other bacteria induced by Tris. J Bacteriol. 1982 Nov;152(2):595–606. doi: 10.1128/jb.152.2.595-606.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Purification and characterization of an N-acylphosphatidylserine from Rhodopseudomonas sphaeroides. Biochemistry. 1982 May 25;21(11):2765–2773. doi: 10.1021/bi00540a029. [DOI] [PubMed] [Google Scholar]
- Drews G., Oelze J. Organization and differentiation of membranes of phototrophic bacteria. Adv Microb Physiol. 1981;22:1–92. doi: 10.1016/s0065-2911(08)60325-2. [DOI] [PubMed] [Google Scholar]
- Dutt A., Dowhan W. Intracellular distribution of enzymes of phospholipid metabolism in several gram-negative bacteria. J Bacteriol. 1977 Oct;132(1):159–165. doi: 10.1128/jb.132.1.159-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federiuk C. S., Shafer J. A. Inactivation of D-serine dehydratase by alkylamines via a transimination of enzyme-linked cofactor. J Biol Chem. 1981 Jul 25;256(14):7416–7423. [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. The relationship of intracytoplasmic membrane assembly to the cell division cycle in Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Mar 25;254(6):1980–1986. [PubMed] [Google Scholar]
- Fraley R. T., Yen G. S., Lueking D. R., Kaplan S. The physical state of the intracytoplasmic membrane of Rhodopseudomonas sphaeroides and its relationship to the cell division cycle. J Biol Chem. 1979 Mar 25;254(6):1987–1991. [PubMed] [Google Scholar]
- Ganong B. R., Raetz C. R. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982 Jan 10;257(1):389–394. [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Larson T. J., Tailleur P., Kantor G. D., Raetz C. R., Bell R. M., Modrich P. Membrane phospholipid synthesis in Escherichia coli. Cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyl/transferase. J Biol Chem. 1980 Oct 10;255(19):9413–9420. [PubMed] [Google Scholar]
- Lueking D. R., Campbell T. B., Burghardt R. C. Light-induced division and genomic synchrony in phototrophically growing cultures of Rhodopseudomonas sphaeroides. J Bacteriol. 1981 May;146(2):790–797. doi: 10.1128/jb.146.2.790-797.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Lueking D. R., Goldfine H. sn-Glycerol-3-phosphate acyltransferase activity in particulate preparations from anaerobic, light-grown cells of Rhodopseudomonas spheroides. Involvement of acyl thiolester derivatives of acyl carrier protein in the synthesis of complex lipids. J Biol Chem. 1975 Nov 10;250(21):8530–8535. [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Characterization of two membrane-associated glycolipids from an Escherichia coli mutant deficient in phosphatidylglycerol. J Biol Chem. 1981 Oct 25;256(20):10690–10696. [PubMed] [Google Scholar]
- Ohta A., Waggoner K., Louie K., Dowhan W. Cloning of genes involved in membrane lipid synthesis. Effects of amplification of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2219–2225. [PubMed] [Google Scholar]
- Onishi J. C., Niederman R. A. Rhodopseudomonas sphaeroides membranes: alterations in phospholipid composition in aerobically and phototrophically grown cells. J Bacteriol. 1982 Mar;149(3):831–839. doi: 10.1128/jb.149.3.831-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol. 1979 Feb;137(2):860–868. doi: 10.1128/jb.137.2.860-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J., Harwood J. L. Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J. 1979 Aug 1;181(2):339–345. doi: 10.1042/bj1810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., KATZ J., BARKER H. A. Cyanide-induced acetylation of amino acids by enzymes of Clostridium kluyveri. J Biol Chem. 1952 Apr;195(2):779–785. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S., Park J. T. Penicillin-binding proteins of Rhodopseudomonas sphaeroides and their membrane localization. J Bacteriol. 1981 Aug;147(2):354–361. doi: 10.1128/jb.147.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber P., Borie R. P., Mikowski E. J., Goldfine H. Phospholipid biosynthesis in some anaerobic bacteria. J Bacteriol. 1981 Jul;147(1):57–61. doi: 10.1128/jb.147.1.57-61.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight C. A., Lueking D. R., Fraley R. T., Kaplan S. Synthesis of photopigments and electron transport components in synchronous phototrophic cultures of Rhodopseudomonas sphaeroides. J Biol Chem. 1978 Jan 25;253(2):465–471. [PubMed] [Google Scholar]



