Abstract
Langerhans cells (LCs) are antigen-presenting cells that reside in the epidermis of the skin and traffic to lymph nodes (LNs). The general role of these cells in skin immune responses is not clear because distinct models of LC depletion resulted in opposite conclusions about their role in contact hypersensitivity (CHS) responses. While comparing these models, we discovered a novel population of LCs that resides in the dermis and does not represent migrating epidermal LCs, as previously thought. Unlike epidermal LCs, dermal Langerin+ dendritic cells (DCs) were radiosensitive and displayed a distinct cell surface phenotype. Dermal Langerin+ DCs migrate from the skin to the LNs after inflammation and in the steady state, and represent the majority of Langerin+ DCs in skin draining LNs. Both epidermal and dermal Langerin+ DCs were depleted by treatment with diphtheria toxin in Lang-DTREGFP knock-in mice. In contrast, transgenic hLang-DTA mice lack epidermal LCs, but have normal numbers of dermal Langerin+ DCs. CHS responses were abrogated upon depletion of both epidermal and dermal LCs, but were unaffected in the absence of only epidermal LCs. This suggests that dermal LCs can mediate CHS and provides an explanation for previous differences observed in the two-model systems.
Langerhans cells (LCs) are professional APCs that form a dense network in the epidermis of the skin. LCs emigrate from the epidermis to skin draining LNs via the dermal lymphatics (1). In the LNs, they display a mature phenotype and can present skin antigens to naive T cells (2, 3). In this way, LCs are thought to initiate adaptive immune responses against the pathogenic organisms that are a constant challenge from the outside world.
One defining characteristic of LCs is the presence of tennis racket–shaped intracellular organelles known as Birbeck granules (4). The development of these Birbeck granules within LCs is dependent on the expression of the endocytic receptor Langerin (CD207), which is a C-type lectin only highly expressed on the surface of LCs (5). Langerin−/− mice lack Birbeck granules, but did not demonstrate defective immune responses, suggesting that the Langerin protein and Birbeck granules are dispensable for immunity to skin pathogens (6). Despite the lack of a role for Langerin in skin immunity, it remains the best marker available for distinguishing LCs from other DC subtypes.
The role of LCs in immune responses to common skin pathogens and irritants is generally unclear. LCs are dispensable for the activation of T cell responses directed against HSV, an infection that is restricted to the epidermis. This was addressed using hematopoietic chimeras, in which it has been shown that LCs are radioresistant and remain of host origin for up to 1 yr (7). In these chimeras, HSV antigens are presented by radiosensitive, LN-resident CD8α+ DCs, and not the radioresistant LCs (8). In addition, although LCs were thought to be the primary APC during skin infection with Leishmania major, recent studies have shown that many other DC subsets, including dermal DCs and CD11b+ LN-resident DCs, can be infected and present L. major antigens (9–12). In light of these findings, the role of LCs in skin immune responses was further debated, and it became necessary to develop better models in which to study LCs.
In an effort to better study the role of LCs in immune responses, mouse models of LC deficiency were developed. Two groups developed knock-in mice in which LCs could be ablated. In these mice, the human diphtheria toxin (DT) receptor (DTR), fused to enhanced GFP (EGFP), was inserted into the endogenous mouse langerin locus. LCs are acutely depleted from these mice after DT administration (13, 14). Another group produced LC-deficient mice by introduction of a bacterial artificial chromosome (BAC) that contains the human Langerin locus and drives constitutive expression of the DTA subunit. These mice lack epidermal LCs in the skin from birth (15). These different models provide systems in which the effect of the absence of LCs can be determined. Contact hypersensitivity (CHS) is one assay used to evaluate cutaneous immune responses. In the acute LC ablation mice, one group observed decreased CHS responses in the absence of LCs (14), whereas the other did not observe any change in CHS after LC depletion (13). Furthermore, in the constitutive LC ablation mice, CHS responses were increased, suggesting a regulatory role for LCs (15). The different results might be caused by differences in CHS technique, in the cell types depleted in each model, or the duration of LC depletion. Thus, rather than defining a precise role for LCs, the conflicting results in LC-deficient mice have further obscured their role in initiation of immune responses to skin antigens.
Upon comparison of hematopoietic chimeras and the different mouse models of LC deficiency, we discovered a novel subset of LCs that are radiosensitive and reside in the dermis of the skin. This population can migrate to skin draining LNs, and it plays a role in the CHS response to hapten.
RESULTS
The majority of LCs in skin draining LNs are not derived from the epidermis
LCs are professional APCs in the epidermis that are distinct from other DCs in that they express high levels of the C-type lectin Langerin (5). Recently, a knock-in mouse was developed in which the fluorescent protein EGFP was inserted into the endogenous Langerin locus (Lang-EGFP) (13). In these mice, EGFP expression reflects expression of Langerin, and is observed in two populations of DCs within skin draining LNs. The first population expresses low levels of Langerin, and high levels of CD8α, and represents blood-derived DCs that do not migrate from the skin (Fig. 1 A) (13). The second population expresses high levels of Langerin and low levels of CD8α, and it is made up of LCs that migrate from the skin. In all further experiments, we will refer only to EGFPhigh, CD8α− DCs as LCs.
Figure 1.
A novel population of radiosensitive LCs in dermis and skin draining LNs. Bone marrow chimeric mice were created by reconstituting lethally irradiated Lang-EGFP mice with bone marrow from congenic Lang-EGFP.SJL donors. (A) Representative flow cytometric analysis of LN CD11c+ I-Ab+ DCs. A broad I-Ab+ gate was used to exclude the small number of non-APCs that express CD11c. The plot shows distinct populations of EGFP−/int, CD8α+ DCs, and EGFPhi CD8α− LCs. Only the latter will be referred to as LCs in this study. (B) Flow cytometric analysis of epidermal (a), dermal (d), and LN DCs (b and c) suspensions from Lang-EGFP.SJL into Lang-EGFP bone marrow chimeric mice. Plots on the right show CD45.1 (donor) and CD45.2 (host) expression on the indicated population. A prominent population of donor-derived LCs is observed in the LNs and dermis, but not in the epidermis.
It has been shown that epidermal LCs are radioresistant and remain of host origin for up to 1 yr in the epidermis of lethally irradiated bone marrow chimeric mice (7). We created bone marrow chimeras to address the radioresistance of different DC populations in skin and skin draining LNs. Lang-EGFP.SJL (CD45.1) donor bone marrow was used to reconstitute lethally irradiated Lang-EGFP (CD45.2) host mice. The resulting chimeric mice were allowed to reconstitute the hematopoietic compartment for at least 6 wk before analysis. As expected, LCs within the epidermis were nearly entirely host derived (Fig. 1 B, gate a). Non-LC DCs within the LNs were almost all donor derived, as expected (Fig. 1 B, gate c). Surprisingly, given the host origin of LCs in the epidermis, the majority of LCs in the skin draining LNs were donor derived (Fig. 1 B, gate b).
The dermis contains a unique population of Langerin+ cells
To better understand the origin of the donor-derived LCs, we analyzed cell suspensions from the dermis of the chimeric mice. The predominant cell in this tissue is a CD11b bright mononuclear phagocyte, as previously concluded from immunofluorescence histology analysis (16). However, a distinct population of MHC class II+ cells are Langerin+ (Fig. 1 B, gate d). Unlike in the epidermis, the majority of EGFP+ LCs were of donor origin. Analysis of multiple chimeras indicated that epidermal LCs were 95% of host origin, but LCs found in the dermis and skin draining LNs were predominantly donor derived (72 and 61%, respectively; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1). Langerin− DCs within the dermis (dermal DCs) were almost entirely donor derived (Fig. S1). Note that both the EGFPlow and EGFP− CD8α+ DCs are also primarily bone marrow derived. Overall, these data suggest that a population of radiosensitive LCs present within the dermis may contribute to the LCs found in skin draining LNs. It was always presumed that dermal Langerin+ cells were epidermal migrants, trafficking through the dermis to access the dermal lymphatics and migrate to the LNs. However, the differences in chimerism between dermal and epidermal LCs suggested that only a minority of the Langerin+ DCs present in the dermis could be migrating epidermal LCs.
In addition to Lang-EGFP knock-in mice, other mouse models have been used to study LCs. The hLang-DTA transgenic mouse was previously described by Kaplan et al., and is a model in which a BAC containing the human Langerin locus drives expression of the DT subunit A (DTA) in LCs (15). hLang-DTA transgenic mice lack epidermal LCs from birth. We crossed hLang-DTA mice to Lang-EGFP mice to determine if all EGFP+ LCs are ablated by the hLangerin-DTA transgene. No EGFP+ cells were found in the epidermis of these mice (Fig. 2 A). However, EGFP+ cells were readily detected in both the dermis and skin draining LNs (Fig. 2 A). Again, such cells are not CD8α-expressing EGFPlow cells previously described, as they express a high level of EGFP and mouse Langerin and no CD8α. Although our results clearly indicate that the human Langerin BAC does not express in precisely the same tissue-specific manner as endogenous mouse Langerin, it conveniently provides for selective elimination of epidermal LCs. Thus, in the absence of migratory epidermal LCs, a population of LCs can be found in both dermis and skin draining LNs. This provides further evidence that the dermal Langerin+ DC population is distinct from migrating epidermal LCs.
Figure 2.
LCs are present in the dermis and skin draining LNs of hLang-DTA mice. (A) Flow cytometry analysis of I-Ab+ CD45.2+ cells in epidermal, dermal, and LN DCs suspensions from Lang-EGFP mice and Lang-EGFP x hLang-DTA Tg mice. In mice expressing the hLang-DTA transgene, EGFP+ cells are absent in epidermal cell suspensions, but present in both dermal and LN DCs preparations. Recovery of LCs in these tissues is ∼50–80% of that found in wild-type Lang-EGFP mice. The SD from two experiments with six Lang-EGFP and three Lang-EGFP x hLang-DTA mice is shown. (B) Immunofluorescence for Langerin and EGFP in cross sections of ear from Lang-EGFP mice and Lang-EGFP x hLang-DTA Tg mice reveals an absence of epidermal LCs (solid arrows) in Lang-EGFP x hLang-DTA Tg mice, whereas dermal Langerin+ DCs (line arrows) are present in both strains. Bar, 75 μm. Representative images from three Lang-EGFP and three Lang-EGFP x hLang-DTA mice are shown.
Immunofluorescence of skin sections also indicated that EGFP+ cells are absent from the epidermis, but clearly present in the dermis of Lang-EGFP x hLang-DTA mice (Fig. 2 B). Within a typical skin section, epidermal LCs are more abundant than dermal Langerin+ DCs (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1). Within the dermis, we observed EGFP+ cells both deep within the dermis and adjacent to hair follicles, which are lined by epidermis (17). To distinguish epidermal LCs within hair follicles from dermal Langerin+ DCs associated with hair follicles, we examined Lang-EGFP x hLang-DTA mice that lack epidermal LCs. Fig. S2 (B–D) shows three different sections of hair follicles. Clearly, dermal Langerin+ DCs are found outside of the epidermis of the hair follicles (Fig. S2, B and C), although in some cases they might be interdigitating within the follicular epidermis (Fig. S2 D). Further work will be necessary to determine if dermal Langerin+ DCs have a distinct anatomical sublocation within the skin and how this may affect their role in skin immunity. We next sought to determine if these two populations could be further distinguished by surface phenotype markers.
Dermal Langerin+ DCs display a distinct phenotype
Epidermal LCs are known to express certain phenotypic markers in addition to Langerin. Two important markers of epidermal LCs are the adhesion molecule Ep-CAM (gp40) (18) and the integrin CD11b (Mac-1) (19). Analysis of EGFP+ cells from the dermis of Lang-EGFP.SJL > Lang EGFP chimeras revealed differences in expression of these epidermal LC markers. Within the epidermis, all LCs are high for both Ep-CAM and CD11b (Fig. 3 A). Furthermore, in the dermis and skin draining LNs, host-derived LCs remained high for both markers. In contrast, donor-derived LCs were predominantly low for both Ep-CAM and CD11b (Fig. 3 A). In addition, in Lang-EGFP x hLang-DTA mice that lack epidermal LCs, none of the EGFP+ cells present in the dermis and skin draining LNs were positive for Ep-CAM and CD11b (Fig. 3 B). Nonchimeric Lang-EGFP mice also exhibit the same two populations (unpublished data). This suggests that Ep-CAM and CD11b can be used to distinguish stable subpopulations of epidermal LCs and dermal Langerin+ DCs in the steady state.
Figure 3.
Donor-derived LCs display a distinct phenotype in dermis and skin draining LNs. Flow cytometry analysis of EGFP+ cells in epidermal, dermal, and LN DC suspensions from Lang-EGFP.SJL into Lang-EGFP bone marrow chimeric mice (A) or Lang-EGFP x hLang-DTA Tg mice (B). Host-derived epidermal DCs are predominantly Ep-CAM+ CD11b+, whereas donor-derived dermal DCs are predominantly CD11b− Ep-CAM−. Data shown are representative of 12 chimeras and 6 Lang-EGFP x hLang-DTA mice.
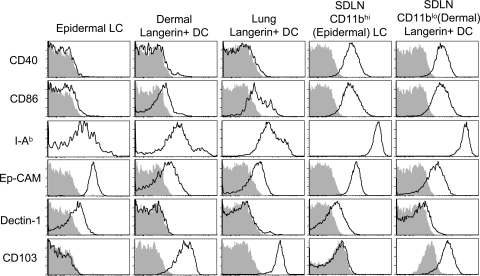
To provide a comprehensive analysis of phenotypic markers on Langerin+ DCs, we analyzed EGFPhi cells from epidermis, dermis, and skin draining LNs (Fig. 4). Importantly, dermal Langerin+ DCs (EGFP+, CD11blow) expressed high levels of mouse Langerin, similar to that observed on epidermal LCs (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1). Both epidermal LCs and dermal Langerin+ DCs expressed low levels of CD40, MHC II (I-Ab), and the costimulatory molecule CD86 (B7-2) in the tissue, but increased levels in the skin draining LNs (Fig. 4). Interestingly, some cell surface molecules, such as Dectin-1 and Ep-CAM, were highly expressed on epidermal LCs, but were absent or low on dermal Langerin+ DCs, both in the tissue and in the draining nodes (Fig. 4). Other molecules, such as CD103, were observed on dermal, but not epidermal LCs, and this was also preserved in the draining nodes. Expression of all analyzed markers on EGFP+CD11b+ cells in the dermis was similar to that of epidermal LCs (unpublished data), which is consistent with these cells being migrating epidermal LCs. We conclude that Langerin+ DCs in the dermis are comprised of both migratory epidermal and dermal populations that display distinct phenotypes. In this regard, we note that the combination of CD103 and CD11b provides a nice distinction of these two populations within Langerin+ DCs in the skin draining LNs (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1).
Figure 4.
Dermal Langerin+ DCs express different levels of costimulatory and adhesion molecules. DCs were harvested from epidermis, dermis, lung, and skin draining LNs (SDLN) of 4 Lang-EGFP animals. CD11c+ populations were enriched from lung and LNs using MACS. Total EGFP+ cells are shown for epidermis and lung. In dermis and skin draining LNs, epidermal LCs and dermal Langerin+ DCs were distinguished using CD103 and CD11b as shown in Fig. S4. Isotype control staining is shown in the shaded histograms. Data are representative of two to six experiments. Fig. S4 is available at http://www.jem.org/cgi/content/full/jem.20071966/DC1.
LCs with a similar phenotype reside in other tissues
It has been reported that a subset of CD103 (αE)-β7 integrin+ and CD11b− DCs within the lung express langerin (20). We analyzed lung tissue from Lang-EGFP and Lang-EGFP x hLang-DTA mice to determine if these cells were present in these mouse models. We identified EGFP+CD8α− LCs in lung tissue of both Lang-EGFP and Lang-EGFP x hLang-DTA mice (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1). The presence of these cells in Lang-EGFP x hLang-DTA mice suggests that they are not related to epidermal LCs, which are absent in these mice. In addition, we found that lung Langerin+ DCs express CD103 and do not express CD11b (Fig. 4 and Fig. S4). As previously mentioned, the use of CD103 and CD11b allowed a clean distinction of two subsets of Langerin+ DCs in the skin draining LNs (Fig. S4). In addition to lung, we were able to detect EGFP+, CD11b−, CD103+ DCs in the liver (unpublished data). Altogether, our results suggest that this novel subset of Langerin+ DCs are present in multiple tissues, most prominent of which is the dermis of the skin.
Dermal Langerin+ DCs migrate to skin draining LNs
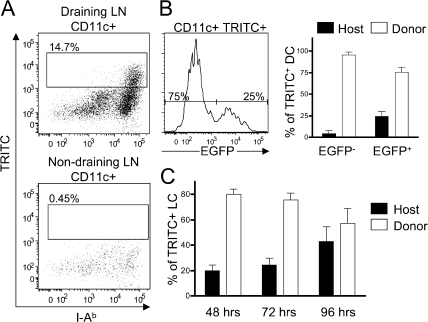
To determine the potential for dermal Langerin+ DCs to be involved in initiating immune responses to skin antigens, it was important to assess the ability of these cells to migrate from the skin to draining LNs. To test this, we applied the fluorescent dye tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC) to the ears of Lang-EGFP.SJL > Lang-EGFP bone marrow chimeric mice. TRITC+ cells begin appearing in the skin draining LNs at 24 h, and were tracked until 96 h. TRITC+ cells were I-Ab high, indicating that the cells were migrant DCs, and the dye was not transferred between cell types within the LNs. In addition, no TRITC+ DCs were observed in nondraining LNs of TRITC-painted chimeras (Fig. 5 A). TRITC+ migrant DCs contained both EGFP− and EGFP+ DCs (Fig. 5 B). TRITC+ EGFP− cells (referred to as dermal DCs) were nearly all donor derived, as expected. However, TRITC+ LCs were also predominantly donor derived (Fig. 5 B). The presence of a high frequency of donor-derived LCs suggests that radiosensitive dermal Langerin+ DCs can migrate from TRITC-painted skin. Application of TRITC is known to induce inflammation within the skin, and can increase migration of epidermal LCs. It was previously shown in Lang-EGFP mice that migration of EGFP+ LCs peaks at 96 h after TRITC painting (13). We observed that at early time points (48/72 h) TRITC+ LCs were predominantly donor derived. However, at 96 h, host-derived LCs comprised almost half of the TRITC+ LCs in the LNs (Fig. 5 C). These results are consistent with early migration of both dermal DCs and dermal Langerin+ DCs, and a later migration of epidermal LCs.
Figure 5.
Donor-derived LCs migrate from the skin to draining LNs. Lang-EGFP.SJL into Lang-EGFP bone marrow chimeras were painted with TRITC on the ear, and draining nodes were analyzed at 48, 72, or 96 h. (A) Dot plots show the fraction of TRITC+ CD11c+ DCs in draining (top) or nondraining (bottom) LNs after 72 h. (B) The histogram (left) shows Langerin (EGFP) expression on the migrated (TRITC+) DCs in the LNs at 72 h. The bar graph (right) shows the mean percentage of donor- (CD45.1+) or host-derived (CD45.2+) cells among migrated LCs (EGFP+) and other DCs (EGFP−). Error bars indicate the SD. (C) Relative percentage of host- and donor-derived EGFP+ LCs in skin draining LNs over time (mean ± the SD) shown for three mice at each time point.
Dermal Langerin+ DCs play a role in CHS responses
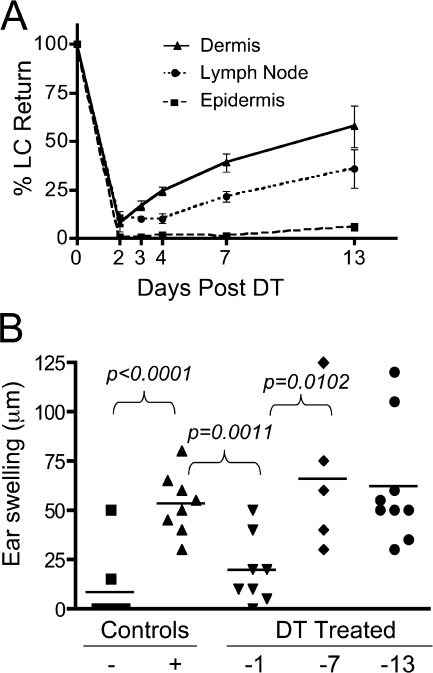
Given the differences in phenotype, anatomical location, and migration kinetics, we sought to determine if dermal Langerin+ DCs and epidermal LCs might have unique functions in immune responses. In this regard, both CD11b+ (epidermal) and CD103+ (dermal) langerin+ DCs isolated from skin draining LNs were able to stimulate T cell proliferation when pulsed with peptide antigen (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20071966/DC1). To examine skin immune responses specifically, we used the CHS assay. We used another Langerin knock-in mouse to test the function of distinct LC subsets in CHS in vivo. Lang-DTREGFP knock-in mice express the human DTR fused to EGFP under the control of the endogenous murine Langerin promoter. Administration of DT to Lang-DTREGFP mice results in ablation of LCs in epidermis and skin draining LNs. It was previously shown in these mice that EGFP+ LCs reappeared in the LNs before reappearing in the epidermis of the skin (13). We reasoned that EGFP+ cells present in the LNs before the return of epidermal LCs might be dermal Langerin+ DCs. Importantly, dermal Langerin+ DCs express similar levels of EGFP and murine Langerin, making these cells equally sensitive to toxin treatment. We administered DT and assessed the return of EGFP+ LCs in epidermis, dermis, and skin draining LNs at intervals for up to 2 wk. At day 2 after toxin treatment, LCs were depleted (>90% reduction) in all tissues. By day 3 after toxin, LCs were returning to the dermis, and continued to increase in frequency over the next 2 wk. LCs did not return to the skin draining LNs within the first 4 d, but were observed at day 7 after DT. Despite return of LCs to both dermis and skin draining LNs, LC return in the epidermis was not observed throughout the time course (Fig. 6 A), which is consistent with previous studies (13, 14). These data provide further correlative evidence that dermal Langerin+ DCs can migrate to skin draining LNs in the steady state. Importantly, the distinct timing of return of dermal Langerin+ DCs and epidermal LCs after toxin treatment provided a window in which to assess their relative roles in CHS responses.
Figure 6.
Dermal Langerin+ DCs contribute to the CHS response. (A) Time course of LC recovery in various tissues. Lang-DTREGFP mice were treated with 1 μg DT i.p. on day 0. The recovery of EGFP+ LCs in epidermis, dermis, and skin draining LNs was determined 2, 3, 4, 7, or 13 d later. The graph shows percentage of LC return, calculated as the percentage of EGFP+ DCs at the time of analysis compared with untreated control mice (mean± the SD) for three mice/group. (B) CHS. Lang-DTREGFP mice were treated with 1 μg DT i.p. at 1, 7, or 13 d before sensitization on the flank with DNFB. Mice were challenged with DNFB on the ear 5 d later, and ear swelling was measured 24 h after challenge. Positive control mice were not DT treated, and received both sensitization and challenge doses. Negative control mice were not sensitized, but received the challenge dose. Experimental results are shown from three independent experiments. The P value for specific comparisons is shown.
It was originally reported that toxin treatment does not impair CHS in Lang-DTREGFP mice when DT is administered 3 d before and 1 d after sensitization (13). However, we recently determined that LC depletion in Lang-DTREGFP mice can reduce CHS responses if the DT is administered 1 d before sensitization with the dinitrofluorobenzene (DNFB) hapten (unpublished data). This depletion strategy provides for ablation of both dermal Langerin+ DCs and epidermal LCs, without return of either population at the time of sensitization. We took advantage of the differential time course of Langerin+ DC return, and administered toxin at either 1 d before sensitization, for depletion of both dermal Langerin+ DCs and epidermal LCs, or 7 or 13 d before sensitization for depletion of only epidermal LCs. CHS responses, as measured by swelling on the challenged ear, were unaffected by toxin administration at 7 or 13 days before sensitization. However, CHS was substantially inhibited by toxin administration 1 d before sensitization (Fig. 6 B). Our results show that Langerin+ cells in general are necessary for an optimal CHS response. Furthermore, they are consistent with the notion that dermal Langerin+ DCs, specifically, can promote this response in the absence of epidermal LCs.
DISCUSSION
A novel population of LCs
We have identified a novel population of LCs that are distinct from the traditional epidermal LCs. This novel population is bone marrow derived in hematopoietic chimeras, whereas epidermal LCs are radioresistant and remain of host origin (7). This LC population is also anatomically distinct, as it occupies the dermis of the skin and is not present in the epidermis. Phenotypically, the dermal Langerin+ DC population is distinct from epidermal LCs in its expression of CD11b, Ep-CAM, Dectin-1, and CD103 (αE)-β7 integrin. Other potential differences between these subsets could be revealed through microarray analysis of sorted populations of dermal Langerin+ DCs and epidermal LCs. We have also identified these cells in the lung and liver, suggesting that they are, in fact, a subset of interstitial DCs found throughout the body.
Dermal Langerin+ DCs can traffic from the skin to draining LNs both in the steady state and after the application of TRITC to the skin. We cannot rule out that these cells can also arrive in the LNs via the blood, but we think it is unlikely, as we do not observe EGFP+ cells in the blood of Lang-EGFP mice. It is probable that these cells have requirements for migration from skin that are similar to epidermal LCs and other DCs. Entry of monocyte precursors, which can give rise to epidermal LCs, has been shown to require CCR2 and CCR6 (7, 21). Although it is likely that dermal Langerin+ DC precursors require CCR2 for entry into the dermis, it is less likely that they require CCR6, which is necessary for entry into the epidermis (22). It seems likely that all DCs would require CCR7 for entry into dermal lymphatics and trafficking to draining LNs (23). Although it is possible that dermal Langerin+ DCs are derived from the same monocyte precursor that has been shown to give rise to epidermal LCs (21), this has not been shown. If this is the case, it is also interesting that in the absence of an inflammatory signal, these LC precursors give rise to dermal Langerin+ DCs, but not epidermal LCs, as shown after depletion of these two subsets with DT administration in Lang-DTREGFP mice. Additional studies will be needed to determine the ontogeny of dermal Langerin+ DCs, and their relationship to epidermal LCs.
Reconciliation of data from different models of LC depletion
Three different models of LC depletion have been described and used to test the role of LCs in CHS responses. Two of these models (Lang-DTREGFP) are knock-in mice in which the human DTR is expressed under the control of the endogenous mouse langerin promoter (13, 14). The third model (hLang-DTA) is a BAC transgenic mouse in which DTA is expressed under the control of the human langerin promoter (15). Disappointingly, these models all came to different conclusions about the role of LCs in CHS responses. The knock-in mice displayed reduced or unchanged CHS after LC depletion, and the BAC transgenic mice had enhanced CHS in the absence of LCs. We presume that the first two models are equivalent, as we have shown a decrease in CHS when LCs were depleted 1 d before sensitization of the mice with DNFB (unpublished data). We show that the timing of depletion is critical for observing decreased CHS, presumably because an additional population of dermal Langerin+ DCs is returning to the skin as little as 3 d after DT administration. The presence of the dermal Langerin+ DC population was not appreciated in previous interpretation of these results. Therefore, we propose that LCs in general are required for maximal CHS responses, but nonepidermal Langerin+ DCs can mediate the CHS response in the absence of epidermal LCs. We also show that dermal Langerin+ DCs are not depleted in hLang-DTA mice, and are therefore likely to mediate CHS in these mice despite the absence of epidermal LCs.
From the available data, it appears that epidermal LCs are not strictly required for CHS. However, they can present antigen and migrate to skin draining LNs. Given their late migration kinetics, relative to dermal DCs, it is tempting to speculate that they serve a negative regulatory function. We did not observe a substantial increase in CHS at the 13-d time point when dermal Langerin+ cells have returned, but epidermal LCs have not (Fig. 6). Thus, our results do not explain the enhancement of CHS responses observed in hLang-DTA mice. It is possible that hLang-DTA mice display enhanced CHS as a result of chronic (lifelong) LC deficiency. Further experiments with these model systems will be necessary to address this question.
The biological role of dermal Langerin+ DCs
It is clear from our studies that dermal Langerin+ DCs are capable of participating in skin immune responses. However, it is not clear if these cells play a role in other responses, including infection. It has recently been shown that gene gun immunization is not affected by depletion of LCs in Lang-DTREGFP mice (24). We have also observed that LCs are not required for epicutaneous immunization in the presence of an adjuvant (unpublished data). In these situations, it is likely that dermal DCs are capable of mediating the response, or that inflammation within the skin leads to recruitment of additional inflammatory APCs that can induce T cell responses after migration to LNs. The ability of dermal Langerin+ DCs to provide a function in CHS that is not redundant with other dermal DCs is surprising, given their anatomical location within the dermis. However, we observed the clustering of these cells around the hair follicles within the dermis. This may provide dermal Langerin+ DCs better access to antigens that do not penetrate deep within the dermis. Alternatively, their functional properties may be related to their expression of Langerin. Langerin is known to bind some virus particles, and it was shown that HIV-1 virus captured by Langerin was internalized into Birbeck granules and degraded (25). We do not know whether dermal Langerin+ DCs display the characteristic Birbeck granules that distinguish epidermal LCs (4). However, the production of these organelles is dependent on Langerin expression (6, 26), which is expressed at similar levels in dermal Langerin+ DCs and epidermal LCs.
Given the observation that these cells are present in the lung, and are important in immune responses, it will be interesting to examine their role in immune responses within the lung. A previous study identified a population of DCs that migrated from the lung and presented viral antigens during influenza infection (27). Additional phenotyping of these migratory lung DCs will be needed to determine if they are, indeed, the lung Langerin+ DC subset we have identified in Lang-EGFP mice.
Our study has identified a novel population of LCs that resides in the dermis of the skin and the lung. This subset is distinct from epidermal LCs, and is capable of contributing to skin immune responses. Identification of this subset provides reconciliation of previous data examining the role of LCs in CHS responses, and insight into the complexity of immune responses to antigens within the skin. These cells should be considered when addressing the role of LCs using any of the models of LC depletion. The immune stimulatory capability of this population also provides another component of the immune system that could be targeted for vaccine development.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) and congenic B6.SJLCD45.1 mice were purchased from the National Cancer Institute. Lang-EGFP and Lang-EGFPDTR knock-in mice were previously described (13), and they were bred to each other to yield mice heterozygous for each knock-in allele. Lang-EGFP mice were also bred to B6.SJLCD45.1 mice to produce Lang-EGFP.SJL mice for chimeras. hLang-DTA mice (15) were backcrossed to B6 for 9 generations and bred to Lang-EGFP mice to produce mice heterozygous for both the EGFP Langerin knock-in reporter and the hLangerin-DTA transgene. All mice were treated in accordance with federal guidelines, and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Radiation bone marrow chimeras.
Single-cell suspensions of bone marrow were prepared and depleted of mature T cells by complement-mediated cytotoxicity with 30H12 (anti-Thy1.2; American Type Culture Collection), as previously described (28). Bone marrow cells were injected i.v. (5 × 106 cells/recipient) into lethally irradiated recipient mice (1000 rads). Hematopoietic chimerism in skin and lymphoid organs was determined by staining with anti-CD45.2 and -CD45.1 antibodies for flow cytometry analysis.
DC preparation from LNs.
DCs from skin draining LNs and lung were prepared by digestion with collagenase D (Sigma-Aldrich) and EDTA, as previously described (29). LN suspension cells were then labeled using MACS anti-CD11c MicroBeads (Miltenyi Biotech) and passed over a magnetized MACS LS selection column. After washing of the magnetized column, CD11c+ DCs were collected from the column.
Preparation of epidermal and dermal cell suspensions.
Epidermal and dermal cell suspensions were prepared from both ear and flank skin. Epidermal cells were prepared by limited trypsinization and dissociation of epidermal sheets by pipetting in DNase, as previously described (30). Dermal cells suspensions were prepared from skin after the removal of the epidermis. Dermal tissue was minced into small pieces and digested for 2 h at 37°C with a solution of RPMI+ 10 mM Hepes+ 0.05% DNase I (Sigma-Aldrich), 0.27% collagenase type XI (Sigma-Aldrich), and 0.027% hyaluronidase type IV-S (Sigma-Aldrich). The absence of epidermal contamination within the dermal cell suspension was confirmed by flow cytometry for dendritic epidermal T cells using an anti-γδTCR antibody (GL3; BD Biosciences). Alternatively, for preservation of trypsin-sensitive epitopes, whole skin was minced and digested as described in the previous section. Dermal Langerin+ cells were identified after this digestion by expression of CD103.
Antibodies and flow cytometry.
Fluorochrome-conjugated antibodies to CD11c, I-Ab, CD11b, CD8α, CD45.1, CD45.2, and CD103, and biotinylated antibodies to CD103, CD40, and CD86 were purchased from eBioscience, BD Biosciences, or BioLegend. Biotinylated antibody to Dectin-1 was purchased from AbD Serotec. Streptavidin-APC obtained from eBioscience or Streptavidin-PerCP obtained from BD Biosciences was used as a secondary antibody with biotinylated antibodies. The anti–Ep-CAM antibody was purified from G8.8 hybridoma supernatant (obtained from A. Farr, University of Washington, Seattle, WA), and biotinylated. Purified anti-mLangerin antibody (929F3) was purchased from AbCys (and is now available from Dendritics), and goat anti–rat IgG (BD Biosciences) was used as the secondary antibody. For intracellular staining of mLangerin, cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences). All data were collected on a BD LSR II flow cytometer and analyzed with FlowJo software (Tree Star, Inc.).
Sorting DC subsets and in vitro stimulation.
DC subsets were sorted after enrichment for CD11c+ cells by MACS (Miltenyi Biotech), using a FACSAria instrument in the Cancer Center Flow Cytometry Core Facility. CD8α+ DCs were sorted as a control population for the stimulation. EGFP+ cells were sorted into dermal Langerin+ DCs (CD103+CD11b−) or epidermal LCs (CD103−CD11b+). Sorted subsets were incubated with SIINFEKL peptide at 10-fold dilutions for 1 h at 37°C, and washed 2 times with media. OT-I CD8 T cells were purified from LN cell suspensions and purified by negative selection using MACS (Miltenyi Biotech). FITC-coupled antibodies to B220, I-Ab, CD4, and CD44 (BD Biosciences or eBioscience) were used, followed by anti-FITC MicroBeads (Miltenyi Biotech). Purified cells were >90% OT-I and were subsequently CFSE labeled; 104 DCs from each sorted subset were incubated at 37°C with 5 × 104 purified CFSE-labeled OT-I T cells. CFSE dye dilution was measured after 65 h.
Immunofluorescence.
Whole ears were fixed in 4% paraformaldehyde in PBS overnight, rinsed with PBS, and infiltrated with 15% sucrose/PBS overnight at 4°C. The medium was changed for 7.5% gelatin containing 15% sucrose at 37°C for 2–4 h, and the tissues were rapidly frozen at −40°C in isopentane (Sigma-Aldrich). Frozen sections were cut at 8 μm and collected on poly-l-lysine–coated slides. Cryostat sections were stained for EGFP and langerin, as follows. After rehydration in PBS, sections were incubated with FITC-conjugated anti-GFP (Rockland Immunochemicals, Inc.) and anti-langerin antibodies (clone: L31; a gift from R. Steinman, The Rockefeller University, New York, NY) (31) for 1 h, followed by anti-FITC–Alexa Fluor 488 (Invitrogen) and anti–rat IgG-Alexa Fluor 546 (Invitrogen). The nuclei were stained with DAPI (Invitrogen). Images were captured using a microscope (DM5500; Leica) with digital system and LAS AF software (version 1.5.1).
TRITC painting.
TRITC was purchased from Invitrogen and prepared at a 10% stock in DMSO. Stock TRITC was diluted to 1% in a 1:1 mix of acetone and dibutylphthalate for painting. 10 μl of 1% TRITC solution was applied per ear, to both the dorsal and ventral sides.
LC depletion with DT.
LCs were systemically depleted in Langerin-DTREGFP mice by i.p. injection of 1 μg DT (List Biological Laboratories), as previously described (13).
CHS.
All mice were shaved at least 1 d before immunization. 25 μl of 0.3% DNFB (Sigma-Aldrich) in a mixture of acetone and olive oil (4:1) was painted on the back flank of the mice. On day 5, all mice were challenged with 5 μl of 0.15% DNFB on both sides of one ear. Ear thickness was measured before and 24 h after challenge, with a spring-loaded micrometer (Mitutoyo).
Online supplemental materials.
Fig. S1 shows that LCs in the dermis and skin draining LNs are predominantly donor derived in bone marrow chimeric mice. Fig. S2 shows dermal Langerin+ DCs in relative frequency and anatomical location. Fig. S3 displays the expression of mLangerin on epidermal LCs and dermal Langerin+ DCs. Fig. S4 demonstrates the expression of CD103 and CD11b on EGFP+ cells in different tissues. Fig. S5 demonstrates the presence of Langerin+ DCs in the lung that display a similar phenotype to dermal Langerin+ DCs. Fig. S6 shows the stimulation of OT-I T cells by pulsed epidermal LCs and dermal Langerin+ DCs. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071966/DC1.
Supplemental Material
Acknowledgments
We thank Steve Jameson and Marc Jenkins for helpful discussion, Xiao-Jie Ding and Brian Goudy for technical assistance, and Paul Champoux for help with flow cytometry sorting.
This work was supported by National Institutes of Health grants PO1 AI35296 (K.A. Hogquist), UO1 AI70380 (Stephen C. Jameson and K.A. Hogquist), and KO8 AR51092 (D.H. Kaplan). The generation and preliminary characterization of the Lang-EGFP and Lang-DTREGFP mice was supported by Agence Nationale de la Recerche (DC in vivo Project).
Abbreviations used: BAC, bacterial artificial chromosome; CHS, contact hypersensitivity; DNFB, dinitrofluorobenzene; DT, diphtheria toxin; DTR, DT receptor; EGFP, enhanced GFP; LC, Langerhans cell; TRITC, tetramethylrhodamine-5-(and-6)-isothiocyanate.
A. Kissenpfennig's present address is Infection and Immunity Group, Centre for Cancer Research and Cell Biology, School of Biomedical Sciences, Queens University, Belfast, Northern Ireland.
References
- 1.Stoitzner, P., K. Pfaller, H. Stossel, and N. Romani. 2002. A close-up view of migrating Langerhans cells in the skin. J. Invest. Dermatol. 118:117–125. [DOI] [PubMed] [Google Scholar]
- 2.Stoitzner, P., S. Holzmann, A.D. McLellan, L. Ivarsson, H. Stossel, M. Kapp, U. Kammerer, P. Douillard, E. Kampgen, F. Koch, et al. 2003. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J. Invest. Dermatol. 120:266–274. [DOI] [PubMed] [Google Scholar]
- 3.Mayerova, D., E.A. Parke, L.S. Bursch, O.A. Odumade, and K.A. Hogquist. 2004. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 21:391–400. [DOI] [PubMed] [Google Scholar]
- 4.Wolff, K. 1967. The fine structure of the Langerhans cell granule. J. Cell Biol. 35:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valladeau, J., O. Ravel, C. Dezutter-Dambuyant, K. Moore, M. Kleijmeer, Y. Liu, V. Duvert-Frances, C. Vincent, D. Schmitt, J. Davoust, et al. 2000. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 12:71–81. [DOI] [PubMed] [Google Scholar]
- 6.Kissenpfennig, A., S. Ait-Yahia, V. Clair-Moninot, H. Stossel, E. Badell, Y. Bordat, J.L. Pooley, T. Lang, E. Prina, I. Coste, et al. 2005. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol. Cell. Biol. 25:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan, R.S., C.M. Smith, G.T. Belz, A.L. van Lint, L.M. Wakim, W.R. Heath, and F.R. Carbone. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 301:1925–1928. [DOI] [PubMed] [Google Scholar]
- 9.Iezzi, G., A. Frohlich, B. Ernst, F. Ampenberger, S. Saeland, N. Glaichenhaus, and M. Kopf. 2006. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J. Immunol. 177:1250–1256. [DOI] [PubMed] [Google Scholar]
- 10.Lemos, M.P., F. Esquivel, P. Scott, and T.M. Laufer. 2004. MHC class II expression restricted to CD8α+ and CD11b+ dendritic cells is sufficient for control of Leishmania major. J. Exp. Med. 199:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misslitz, A.C., K. Bonhagen, D. Harbecke, C. Lippuner, T. Kamradt, and T. Aebischer. 2004. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur. J. Immunol. 34:715–725. [DOI] [PubMed] [Google Scholar]
- 12.Ritter, U., A. Meissner, C. Scheidig, and H. Korner. 2004. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 34:1542–1550. [DOI] [PubMed] [Google Scholar]
- 13.Kissenpfennig, A., S. Henri, B. Dubois, C. Laplace-Builhe, P. Perrin, N. Romani, C.H. Tripp, P. Douillard, L. Leserman, D. Kaiserlian, et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654. [DOI] [PubMed] [Google Scholar]
- 14.Bennett, C.L., E. van Rijn, S. Jung, K. Inaba, R.M. Steinman, M.L. Kapsenberg, and B.E. Clausen. 2005. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan, D.H., M.C. Jenison, S. Saeland, W.D. Shlomchik, and M.J. Shlomchik. 2005. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 23:611–620. [DOI] [PubMed] [Google Scholar]
- 16.Dupasquier, M., P. Stoitzner, A. van Oudenaren, N. Romani, and P.J. Leenen. 2004. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J. Invest. Dermatol. 123:876–879. [DOI] [PubMed] [Google Scholar]
- 17.Paus, R., C. van der Veen, S. Eichmuller, T. Kopp, E. Hagen, S. Muller-Rover, and U. Hofmann. 1998. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Invest. Dermatol. 111:7–18. [DOI] [PubMed] [Google Scholar]
- 18.Borkowski, T.A., A.J. Nelson, A.G. Farr, and M.C. Udey. 1996. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur. J. Immunol. 26:110–114. [DOI] [PubMed] [Google Scholar]
- 19.Douillard, P., P. Stoitzner, C.H. Tripp, V. Clair-Moninot, S. Ait-Yahia, A.D. McLellan, A. Eggert, N. Romani, and S. Saeland. 2005. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J. Invest. Dermatol. 125:983–994. [DOI] [PubMed] [Google Scholar]
- 20.Sung, S.S., S.M. Fu, C.E. Rose Jr., F. Gaskin, S.T. Ju, and S.R. Beaty. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176:2161–2172. [DOI] [PubMed] [Google Scholar]
- 21.Ginhoux, F., F. Tacke, V. Angeli, M. Bogunovic, M. Loubeau, X.M. Dai, E.R. Stanley, G.J. Randolph, and M. Merad. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charbonnier, A.S., N. Kohrgruber, E. Kriehuber, G. Stingl, A. Rot, and D. Maurer. 1999. Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal langerhans cells. J. Exp. Med. 190:1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohl, L., M. Mohaupt, N. Czeloth, G. Hintzen, Z. Kiafard, J. Zwirner, T. Blankenstein, G. Henning, and R. Forster. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 21:279–288. [DOI] [PubMed] [Google Scholar]
- 24.Stoecklinger, A., I. Grieshuber, S. Scheiblhofer, R. Weiss, U. Ritter, A. Kissenpfennig, B. Malissen, N. Romani, F. Koch, F. Ferreira, et al. 2007. Epidermal langerhans cells are dispensable for humoral and cell-mediated immunity elicited by gene gun immunization. J. Immunol. 179:886–893. [DOI] [PubMed] [Google Scholar]
- 25.de Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M.A. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T.B. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367–371. [DOI] [PubMed] [Google Scholar]
- 26.Valladeau, J., C. Dezutter-Dambuyant, and S. Saeland. 2003. Langerin/CD207 sheds light on formation of birbeck granules and their possible function in Langerhans cells. Immunol. Res. 28:93–107. [DOI] [PubMed] [Google Scholar]
- 27.Belz, G.T., C.M. Smith, L. Kleinert, P. Reading, A. Brooks, K. Shortman, F.R. Carbone, and W.R. Heath. 2004. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA. 101:8670–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolic-Zugic, J., and M.J. Bevan. 1988. Thymocytes expressing CD8 differentiate into CD4+ cells following intrathymic injection. Proc. Natl. Acad. Sci. USA. 85:8633–8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vremec, D., M. Zorbas, R. Scollay, D.J. Saunders, C.F. Ardavin, L. Wu, and K. Shortman. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borkowski, T.A., J.J. Letterio, C.L. Mackall, A. Saitoh, X.J. Wang, D.R. Roop, R.E. Gress, and M.C. Udey. 1997. A role for TGFbeta1 in langerhans cell biology. Further characterization of the epidermal Langerhans cell defect in TGFbeta1 null mice. J. Clin. Invest. 100:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheong, C., J. Idoyaga, Y. Do, M. Pack, S.H. Park, H. Lee, Y.S. Kang, J.H. Choi, J.Y. Kim, A. Bonito, et al. 2007. Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207. J. Immunol. Methods. 324:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.