Abstract
This study examined the association between 17p allelic loss, p53 gene mutation, p53 protein expression and DNA aneuploidy in a series of adenocarcinomas arising in the oesophagus and gastric cardia. 17p allelic loss was detected in 79% (15 of 19) of oesophageal and in 83% (29 of 35) of gastric adenocarcinomas. p53 mutations were detected in 70% (14 of 20) and 63% (26 of 41) of oesophageal and of gastric adenocarcinomas respectively. Both tumour types were associated with a predominance of base transitions at CpG dinucleotides. In five cases of oesophageal adenocarcinoma, the same mutation was detected both in tumour and in adjacent dysplastic Barrett's epithelium. Diffuse p53 protein expression was detected in 65% (13 of 20) and 59% (24 of 41) of oesophageal and of gastric tumours, respectively, and was associated with the presence of p53 missense mutation (Chi-squared, P < 0.0001). DNA aneuploidy was detected in 80% (16 of 20) of oesophageal and in 70% (28 of 40) of gastric tumours. No association was found between p53 or DNA content abnormalities and tumour stage or histological subtype. In conclusion, this study detected a similar pattern of p53 alterations in adenocarcinoma of the oesophagus and gastric cardia--molecular data consistent with the observation that these tumours demonstrate similar clinical and epidemiological features.
Full text
PDF




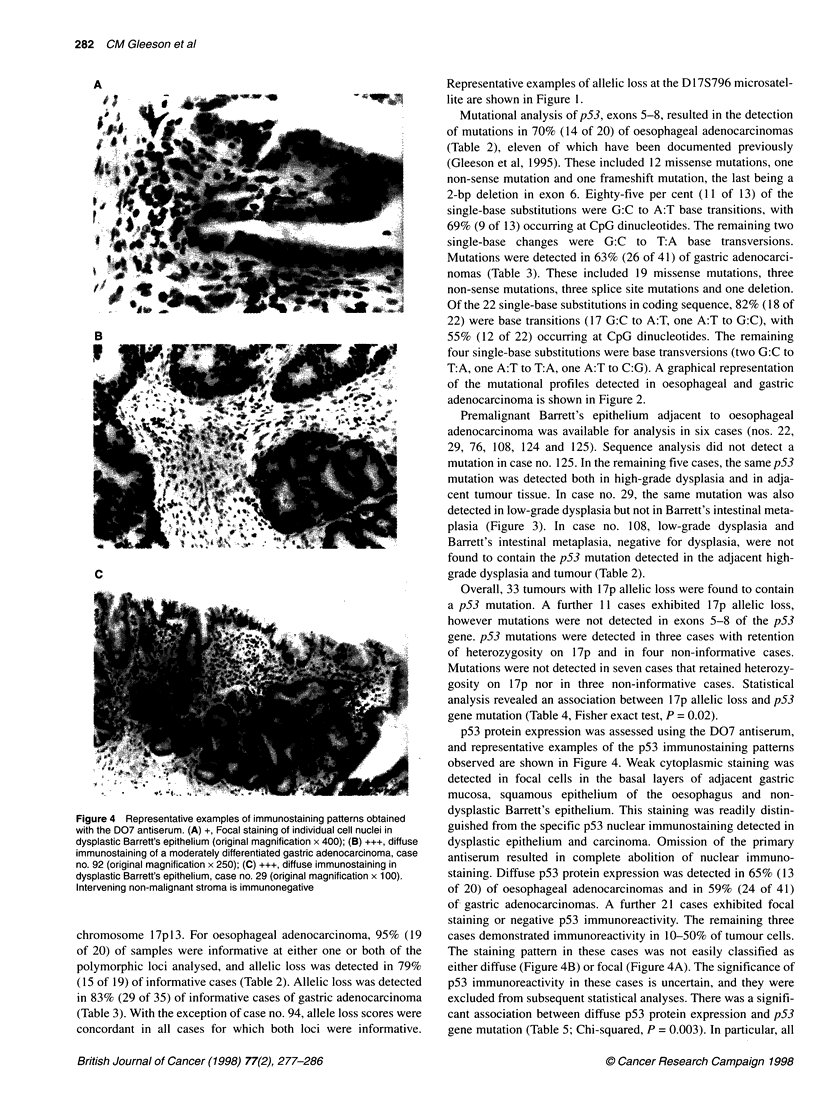




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Blot W. J., Devesa S. S., Kneller R. W., Fraumeni J. F., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991 Mar 13;265(10):1287–1289. [PubMed] [Google Scholar]
- Blount P. L., Galipeau P. C., Sanchez C. A., Neshat K., Levine D. S., Yin J., Suzuki H., Abraham J. M., Meltzer S. J., Reid B. J. 17p allelic losses in diploid cells of patients with Barrett's esophagus who develop aneuploidy. Cancer Res. 1994 May 1;54(9):2292–2295. [PubMed] [Google Scholar]
- Blount P. L., Meltzer S. J., Yin J., Huang Y., Krasna M. J., Reid B. J. Clonal ordering of 17p and 5q allelic losses in Barrett dysplasia and adenocarcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3221–3225. doi: 10.1073/pnas.90.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P. L., Ramel S., Raskind W. H., Haggitt R. C., Sanchez C. A., Dean P. J., Rabinovitch P. S., Reid B. J. 17p allelic deletions and p53 protein overexpression in Barrett's adenocarcinoma. Cancer Res. 1991 Oct 15;51(20):5482–5486. [PubMed] [Google Scholar]
- Brito M. J., Filipe M. I., Williams G. T., Thompson H., Ormerod M. G., Titley J. DNA ploidy in early gastric carcinoma (T1): a flow cytometric study of 100 European cases. Gut. 1993 Feb;34(2):230–234. doi: 10.1136/gut.34.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carder P., Wyllie A. H., Purdie C. A., Morris R. G., White S., Piris J., Bird C. C. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. Oncogene. 1993 May;8(5):1397–1401. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Cripps K. J., Purdie C. A., Carder P. J., White S., Komine K., Bird C. C., Wyllie A. H. A study of stabilisation of p53 protein versus point mutation in colorectal carcinoma. Oncogene. 1994 Sep;9(9):2739–2743. [PubMed] [Google Scholar]
- Fléjou J. F., Muzeau F., Potet F., Lepelletier F., Fékété F., Hénin D. Overexpression of the p53 tumor suppressor gene product in esophageal and gastric carcinomas. Pathol Res Pract. 1994 Dec;190(12):1141–1148. doi: 10.1016/S0344-0338(11)80440-1. [DOI] [PubMed] [Google Scholar]
- Fléjou J. F., Potet F., Muzeau F., Le Pelletier F., Fékété F., Hénin D. Overexpression of p53 protein in Barrett's syndrome with malignant transformation. J Clin Pathol. 1993 Apr;46(4):330–333. doi: 10.1136/jcp.46.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson C. M., Sloan J. M., McGuigan J. A., Ritchie A. J., Russell S. E. Base transitions at CpG dinucleotides in the p53 gene are common in esophageal adenocarcinoma. Cancer Res. 1995 Aug 1;55(15):3406–3411. [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Haggitt R. C., Reid B. J., Rabinovitch P. S., Rubin C. E. Barrett's esophagus. Correlation between mucin histochemistry, flow cytometry, and histologic diagnosis for predicting increased cancer risk. Am J Pathol. 1988 Apr;131(1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Hamelin R., Fléjou J. F., Muzeau F., Potet F., Laurent-Puig P., Fékété F., Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994 Oct;107(4):1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Hardwick R. H., Shepherd N. A., Moorghen M., Newcomb P. V., Alderson D. Adenocarcinoma arising in Barrett's oesophagus: evidence for the participation of p53 dysfunction in the dysplasia/carcinoma sequence. Gut. 1994 Jun;35(6):764–768. doi: 10.1136/gut.35.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hongyo T., Buzard G. S., Palli D., Weghorst C. M., Amorosi A., Galli M., Caporaso N. E., Fraumeni J. F., Jr, Rice J. M. Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res. 1995 Jun 15;55(12):2665–2672. [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Huang Y., Boynton R. F., Blount P. L., Silverstein R. J., Yin J., Tong Y., McDaniel T. K., Newkirk C., Resau J. H., Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992 Dec 1;52(23):6525–6530. [PubMed] [Google Scholar]
- Johnson H., Jr, Belluco C., Masood S., Abou-Azama A. M., Kahn L., Wise L. The value of flow cytometric analysis in patients with gastric cancer. Arch Surg. 1993 Mar;128(3):314–317. doi: 10.1001/archsurg.1993.01420150070013. [DOI] [PubMed] [Google Scholar]
- Kalish R. J., Clancy P. E., Orringer M. B., Appelman H. D. Clinical, epidemiologic, and morphologic comparison between adenocarcinomas arising in Barrett's esophageal mucosa and in the gastric cardia. Gastroenterology. 1984 Mar;86(3):461–467. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Krishnadath K. K., Tilanus H. W., van Blankenstein M., Bosman F. T., Mulder A. H. Accumulation of p53 protein in normal, dysplastic, and neoplastic Barrett's oesophagus. J Pathol. 1995 Feb;175(2):175–180. doi: 10.1002/path.1711750204. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Martin H. M., Filipe M. I., Morris R. W., Lane D. P., Silvestre F. p53 expression and prognosis in gastric carcinoma. Int J Cancer. 1992 Apr 1;50(6):859–862. doi: 10.1002/ijc.2910500604. [DOI] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nekarda H., Hoelscher A. H., Bollschweiler E., Harbeck N., Becker K., Siewert J. R., Harbec N [corrected to Harbeck N. ]. Prognostic value of DNA ploidy and c-erbB-2 oncoprotein overexpression in adenocarcinoma of Barrett's esophagus. Cancer. 1994 Apr 1;73(7):1785–1794. doi: 10.1002/1097-0142(19940401)73:7<1785::aid-cncr2820730703>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Neshat K., Sanchez C. A., Galipeau P. C., Blount P. L., Levine D. S., Joslyn G., Reid B. J. p53 mutations in Barrett's adenocarcinoma and high-grade dysplasia. Gastroenterology. 1994 Jun;106(6):1589–1595. doi: 10.1016/0016-5085(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Offerhaus G. J., De Feyter E. P., Cornelisse C. J., Tersmette K. W., Floyd J., Kern S. E., Vogelstein B., Hamilton S. R. The relationship of DNA aneuploidy to molecular genetic alterations in colorectal carcinoma. Gastroenterology. 1992 May;102(5):1612–1619. doi: 10.1016/0016-5085(92)91721-f. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Wright E. C., Swensen J., Gruis N. A., Goldgar D., Skolnick M. H. Dinucleotide repeat polymorphism at the D17S514 locus. Nucleic Acids Res. 1991 Sep 11;19(17):4794–4794. [PMC free article] [PubMed] [Google Scholar]
- Powell J., McConkey C. C. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990 Sep;62(3):440–443. doi: 10.1038/bjc.1990.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani G. N., Renault B., Pellegata N. S., Fattorini P., Magni E., Bacci F., Amadori D. Loss of heterozygosity and K-ras gene mutations in gastric cancer. Hum Genet. 1993 Oct 1;92(3):244–249. doi: 10.1007/BF00244466. [DOI] [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Rabinovitch P. S. Barrett's esophagus. Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987 Jul;93(1):1–11. [PubMed] [Google Scholar]
- Renault B., van den Broek M., Fodde R., Wijnen J., Pellegata N. S., Amadori D., Khan P. M., Ranzani G. N. Base transitions are the most frequent genetic changes at P53 in gastric cancer. Cancer Res. 1993 Jun 1;53(11):2614–2617. [PubMed] [Google Scholar]
- Sano T., Tsujino T., Yoshida K., Nakayama H., Haruma K., Ito H., Nakamura Y., Kajiyama G., Tahara E. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res. 1991 Jun 1;51(11):2926–2931. [PubMed] [Google Scholar]
- Seruca R., David L., Holm R., Nesland J. M., Fangan B. M., Castedo S., Sobrinho-Simões M., Børresen A. L. P53 mutations in gastric carcinomas. Br J Cancer. 1992 May;65(5):708–710. doi: 10.1038/bjc.1992.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao Y. H., Rugge M., Correa P., Lehmann H. P., Scheer W. D. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994 Mar;144(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Sidoni A., Lancia D., Pietropaoli N., Ferri I. Changing patterns in gastric carcinoma. Tumori. 1989 Dec 31;75(6):605–608. doi: 10.1177/030089168907500619. [DOI] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Spechler S. J., Robbins A. H., Rubins H. B., Vincent M. E., Heeren T., Doos W. G., Colton T., Schimmel E. M. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984 Oct;87(4):927–933. [PubMed] [Google Scholar]
- Thilly W. G. Mutational spectrometry in animal toxicity testing. Annu Rev Pharmacol Toxicol. 1990;30:369–385. doi: 10.1146/annurev.pa.30.040190.002101. [DOI] [PubMed] [Google Scholar]
- Thompson J. J., Zinsser K. R., Enterline H. T. Barrett's metaplasia and adenocarcinoma of the esophagus and gastroesophageal junction. Hum Pathol. 1983 Jan;14(1):42–61. doi: 10.1016/s0046-8177(83)80045-8. [DOI] [PubMed] [Google Scholar]
- Uchino S., Noguchi M., Ochiai A., Saito T., Kobayashi M., Hirohashi S. p53 mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer. 1993 Jul 9;54(5):759–764. doi: 10.1002/ijc.2910540509. [DOI] [PubMed] [Google Scholar]
- Wang H. H., Antonioli D. A., Goldman H. Comparative features of esophageal and gastric adenocarcinomas: recent changes in type and frequency. Hum Pathol. 1986 May;17(5):482–487. doi: 10.1016/s0046-8177(86)80038-7. [DOI] [PubMed] [Google Scholar]
- Winters C., Jr, Spurling T. J., Chobanian S. J., Curtis D. J., Esposito R. L., Hacker J. F., 3rd, Johnson D. A., Cruess D. F., Cotelingam J. D., Gurney M. S. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987 Jan;92(1):118–124. [PubMed] [Google Scholar]
- Wu X., Bayle J. H., Olson D., Levine A. J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993 Jul;7(7A):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Yonemura Y., Ohoyama S., Kimura H., Matumoto H., Ninomiya I., Kosaka T., Yamaguchi A., Miwa K., Miyazaki I. Independent clinical and flow cytometric prognostic factors for the survival of patients with stage I gastric cancer. Surg Today. 1992;22(5):416–420. doi: 10.1007/BF00308790. [DOI] [PubMed] [Google Scholar]




