Abstract
The human multidrug resistance protein (MRP1) confers resistance of cells to a number of different cytostatic drugs and functions as an export pump for glutathione S-conjugates, glucuronides and other amphiphilic anions. The present study details for the first time MRP1-mediated ATP-dependent transport of various glutathione S-conjugates of the bifunctional alkylating agents chlorambucil and melphalan. In membrane vesicles prepared from cells expressing recombinant MRP1, the conjugates were transported at rates in the following order: monoglutathionyl chlorambucil > bisglutathionyl chlorambucil > monohydroxy monoglutathionyl chlorambucil and monoglutathionyl melphalan > monohydroxy monoglutathionyl melphalan. In addition, we show that membranes from chlorambucil-resistant GST-alpha-overexpressing CHO cells as well as from their parental cells express the hamster homologue of MRP1. With both CHO cell membrane preparations, we observed ATP-dependent transport of monoglutathionyl chlorambucil and of leukotriene C4, a glutathione S-conjugate and high-affinity substrate of MRP1. The transport rates measured in the resistant cells were only two- to three-fold higher than those measured in the control cells. These results together with cytotoxicity assays comparing MRP1-overexpressing cell pairs with the CHO cell pair indicate that, although MRP1-mediated transport is active, it may not be the rate-limiting step in chlorambucil resistance in these cell lines.
Full text
PDF


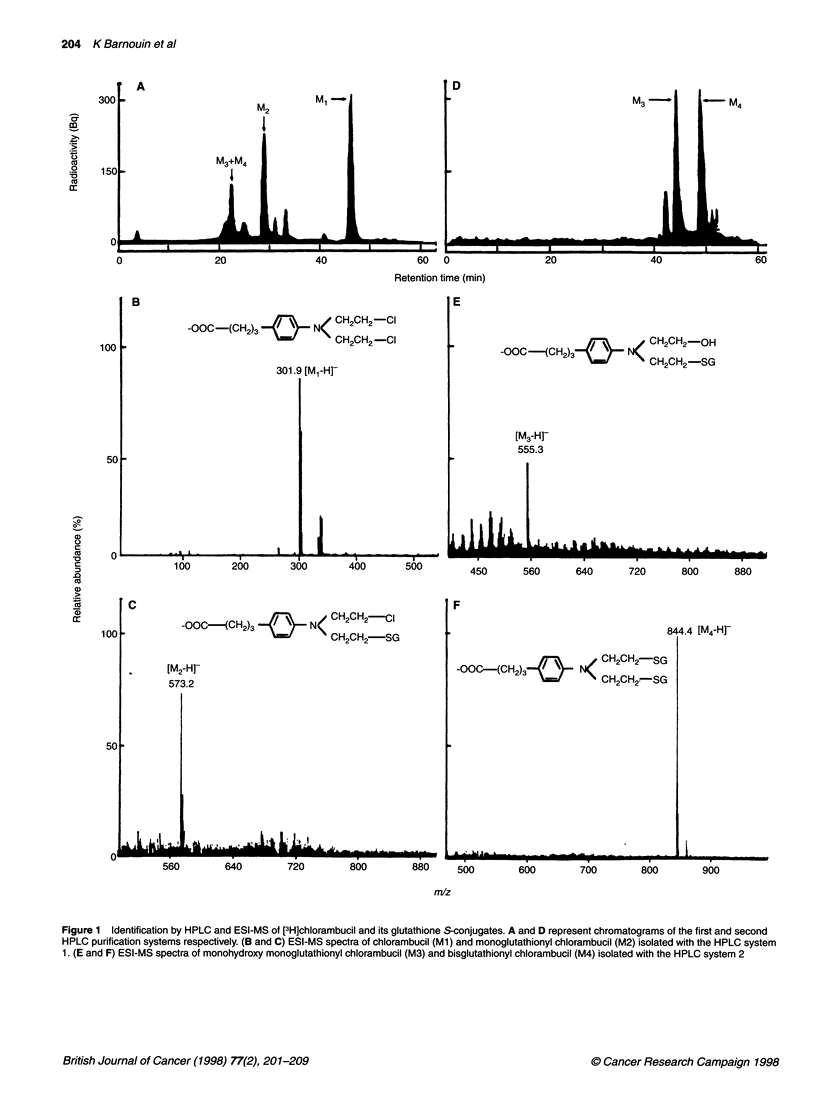
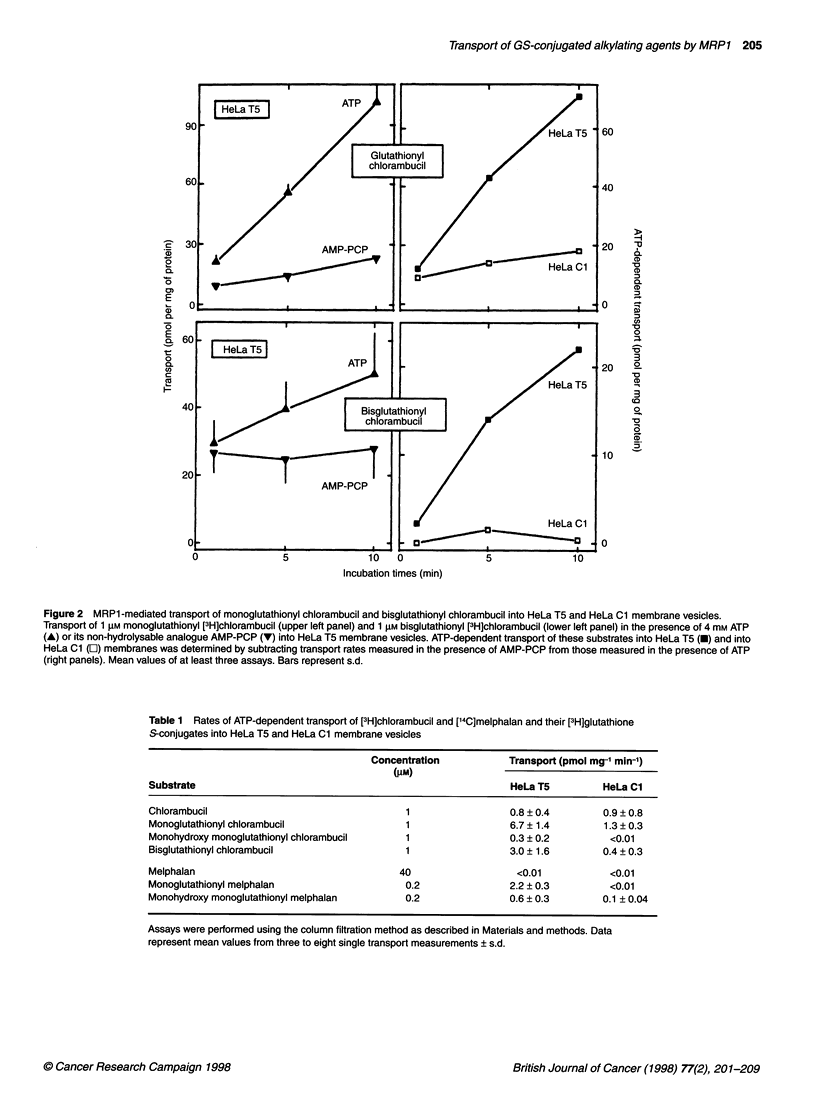
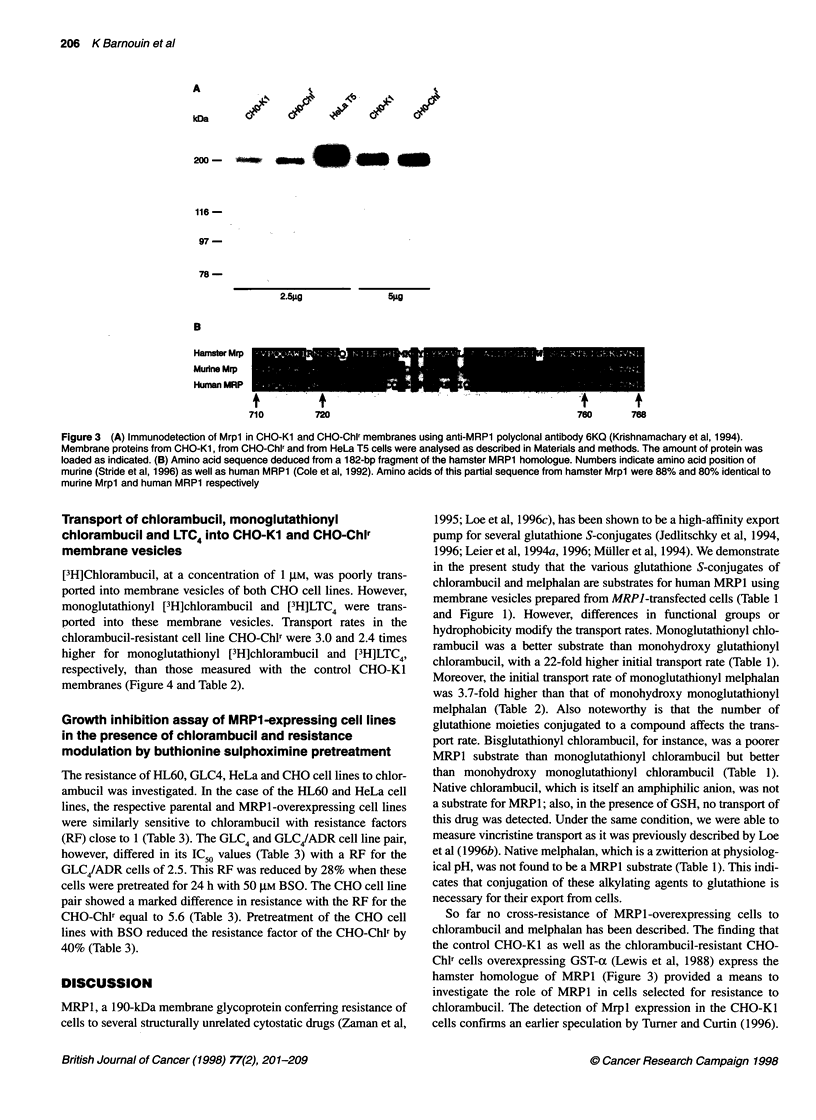
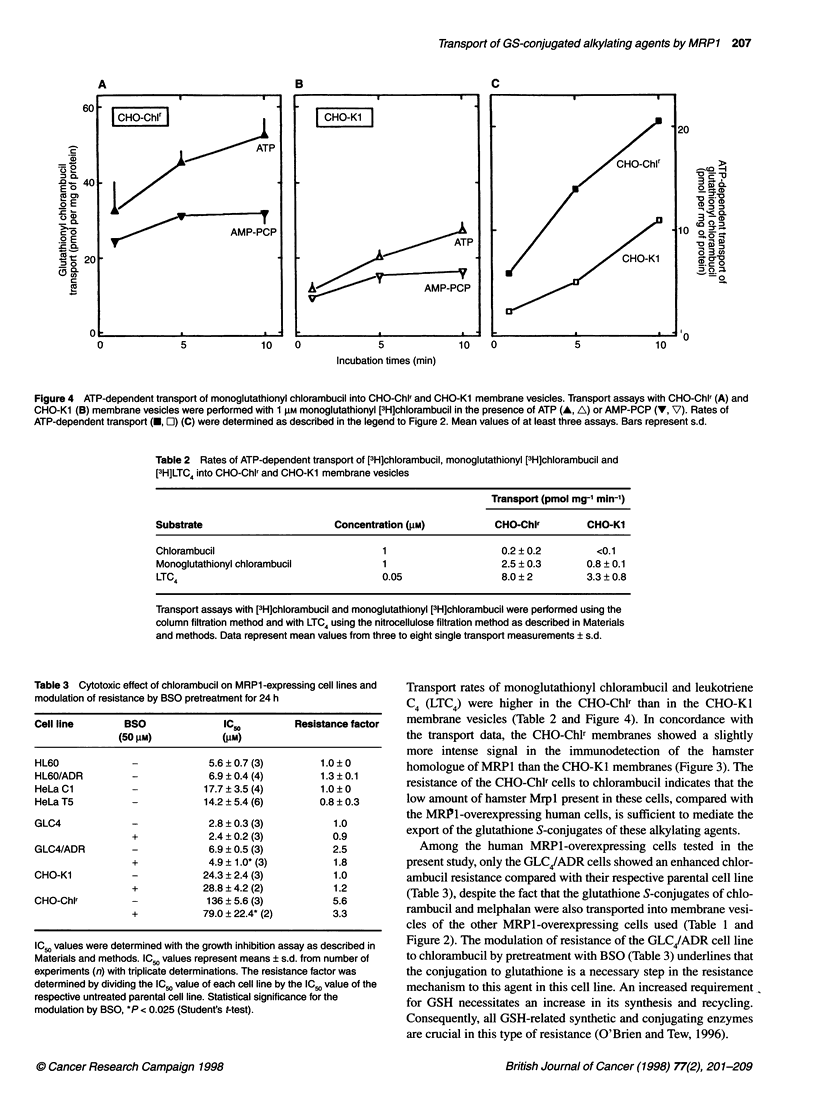


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Transport of glutathione disulfide and glutathione S-conjugates in hepatocyte plasma membrane vesicles. Methods Enzymol. 1994;233:416–425. doi: 10.1016/s0076-6879(94)33048-4. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Goldenberg G. J. Uptake and decomposition of chlorambucil by L5178Y lymphoblasts in vitro. Biochem Pharmacol. 1983 Feb 1;32(3):535–539. doi: 10.1016/0006-2952(83)90535-x. [DOI] [PubMed] [Google Scholar]
- Böhme M., Büchler M., Müller M., Keppler D. Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS Lett. 1993 Oct 25;333(1-2):193–196. doi: 10.1016/0014-5793(93)80403-h. [DOI] [PubMed] [Google Scholar]
- Büchler M., Böhme M., Ortlepp H., Keppler D. Functional reconstitution of ATP-dependent transporters from the solubilized hepatocyte canalicular membrane. Eur J Biochem. 1994 Sep 1;224(2):345–352. doi: 10.1111/j.1432-1033.1994.00345.x. [DOI] [PubMed] [Google Scholar]
- Büchler M., König J., Brom M., Kartenbeck J., Spring H., Horie T., Keppler D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996 Jun 21;271(25):15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. The spontaneous and glutathione S-transferase-mediated reaction of chlorambucil with glutathione. Cancer Commun. 1990;2(8):279–285. doi: 10.3727/095535490820874263. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Dulik D. M., Colvin O. M., Fenselau C. Characterization of glutathione conjugates of chlorambucil by fast atom bombardment and thermospray liquid chromatography/mass spectrometry. Biomed Environ Mass Spectrom. 1990 Apr;19(4):248–252. doi: 10.1002/bms.1200190408. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Pulford D. J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Barnouin K., Kurz G., Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996 Mar 1;56(5):988–994. [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994 Sep 15;54(18):4833–4836. [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Krishnamachary N., Ma L., Zheng L., Safa A. R., Center M. S. Analysis of MRP gene expression and function in HL60 cells isolated for resistance to adriamycin. Oncol Res. 1994;6(3):119–127. [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Center M., Cole S. P., Deeley R. G., Keppler D. ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J. 1996 Mar 1;314(Pt 2):433–437. doi: 10.1042/bj3140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Cole S. P., Deeley R. G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994 Nov 11;269(45):27807–27810. [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Keppler D. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells. Eur J Biochem. 1994 Mar 1;220(2):599–606. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe D. W., Almquist K. C., Cole S. P., Deeley R. G. ATP-dependent 17 beta-estradiol 17-(beta-D-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J Biol Chem. 1996 Apr 19;271(16):9683–9689. doi: 10.1074/jbc.271.16.9683. [DOI] [PubMed] [Google Scholar]
- Loe D. W., Almquist K. C., Deeley R. G., Cole S. P. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996 Apr 19;271(16):9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- Loe D. W., Deeley R. G., Cole S. P. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1996 Jun;32A(6):945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Mayer R., Kartenbeck J., Büchler M., Jedlitschky G., Leier I., Keppler D. Expression of the MRP gene-encoded conjugate export pump in liver and its selective absence from the canalicular membrane in transport-deficient mutant hepatocytes. J Cell Biol. 1995 Oct;131(1):137–150. doi: 10.1083/jcb.131.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Gilmore K. S., Harris J. M., Hartley J. A., Ketterer B. Chlorambucil-monoglutathionyl conjugate is sequestered by human alpha class glutathione S-transferases. Br J Cancer. 1992 Sep;66(3):433–438. doi: 10.1038/bjc.1992.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M. L., Tew K. D. Glutathione and related enzymes in multidrug resistance. Eur J Cancer. 1996 Jun;32A(6):967–978. doi: 10.1016/0959-8049(96)00051-2. [DOI] [PubMed] [Google Scholar]
- Pourtier-Manzanedo A., Boesch D., Loor F. FK-506 (fujimycin) reverses the multidrug resistance of tumor cells in vitro. Anticancer Drugs. 1991 Jun;2(3):279–283. doi: 10.1097/00001813-199106000-00010. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Alexander J., Harris A. L., Hickson I. D. Isolation and characterization of a Chinese hamster ovary cell line resistant to bifunctional nitrogen mustards. Cancer Res. 1986 Dec;46(12 Pt 1):6290–6294. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride B. D., Valdimarsson G., Gerlach J. H., Wilson G. M., Cole S. P., Deeley R. G. Structure and expression of the messenger RNA encoding the murine multidrug resistance protein, an ATP-binding cassette transporter. Mol Pharmacol. 1996 Jun;49(6):962–971. [PubMed] [Google Scholar]
- Turner R. N., Curtin N. J. Dipyridamole increases VP16 growth inhibition, accumulation and retention in parental and multidrug-resistant CHO cells. Br J Cancer. 1996 Apr;73(7):856–860. doi: 10.1038/bjc.1996.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistica D. T. Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther. 1983;22(3):379–406. doi: 10.1016/0163-7258(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Voyksner R. D. Electrospray LC/MS--can it be used to determine lower molecular weight molecules? Nature. 1992 Mar 5;356(6364):86–87. doi: 10.1038/356086a0. [DOI] [PubMed] [Google Scholar]
- Wilm M., Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996 Jan 1;68(1):1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- Zaman G. J., Lankelma J., van Tellingen O., Beijnen J., Dekker H., Paulusma C., Oude Elferink R. P., Baas F., Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Mulder N. H. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987 Apr 1;47(7):1780–1784. [PubMed] [Google Scholar]



