Abstract
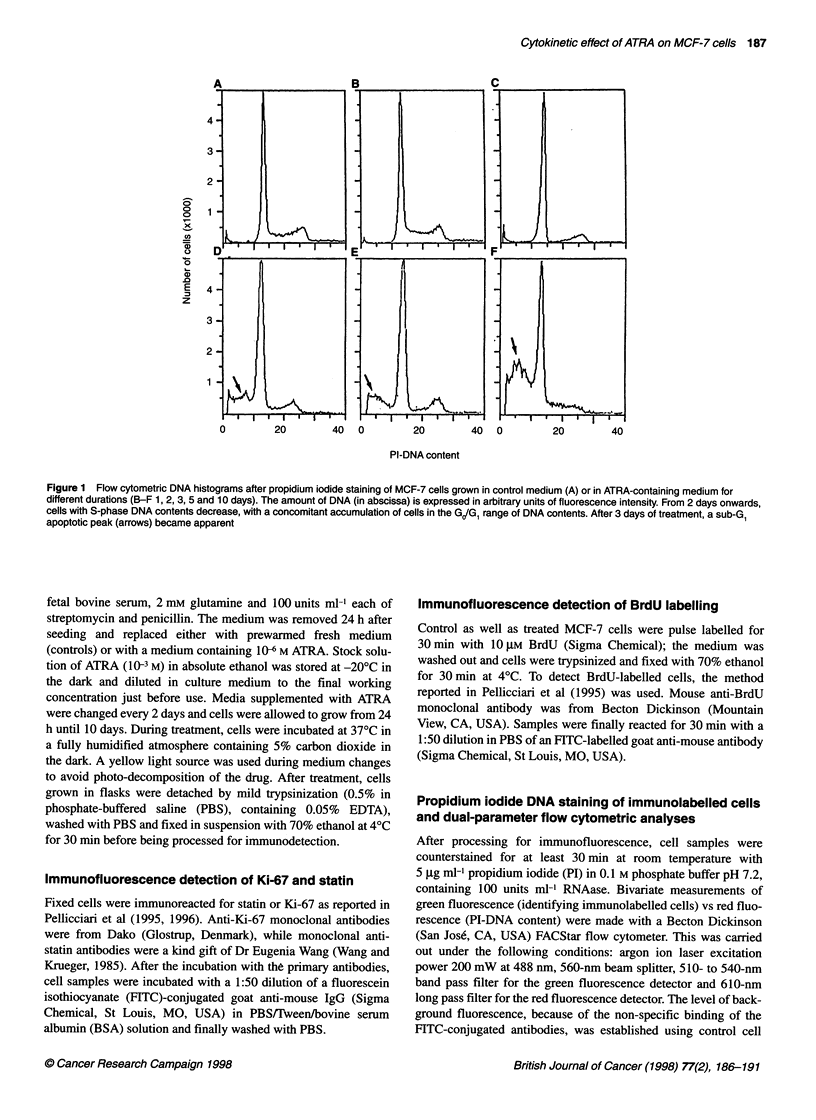
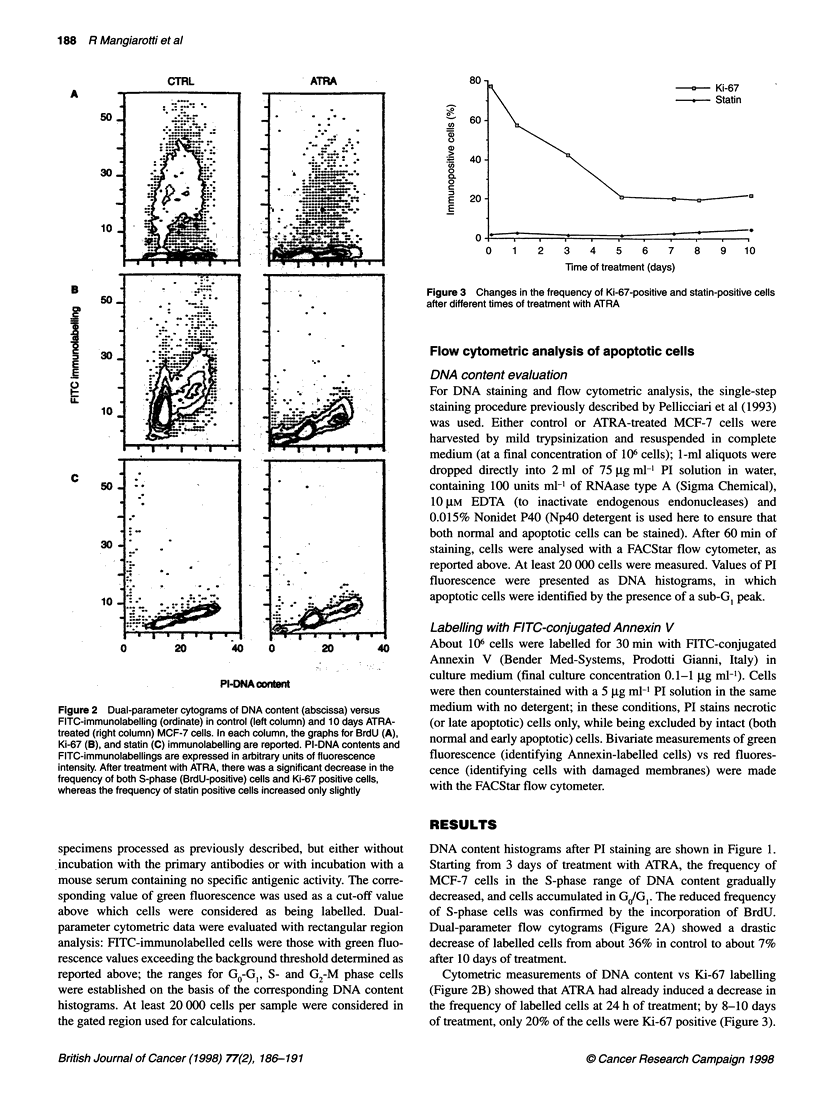
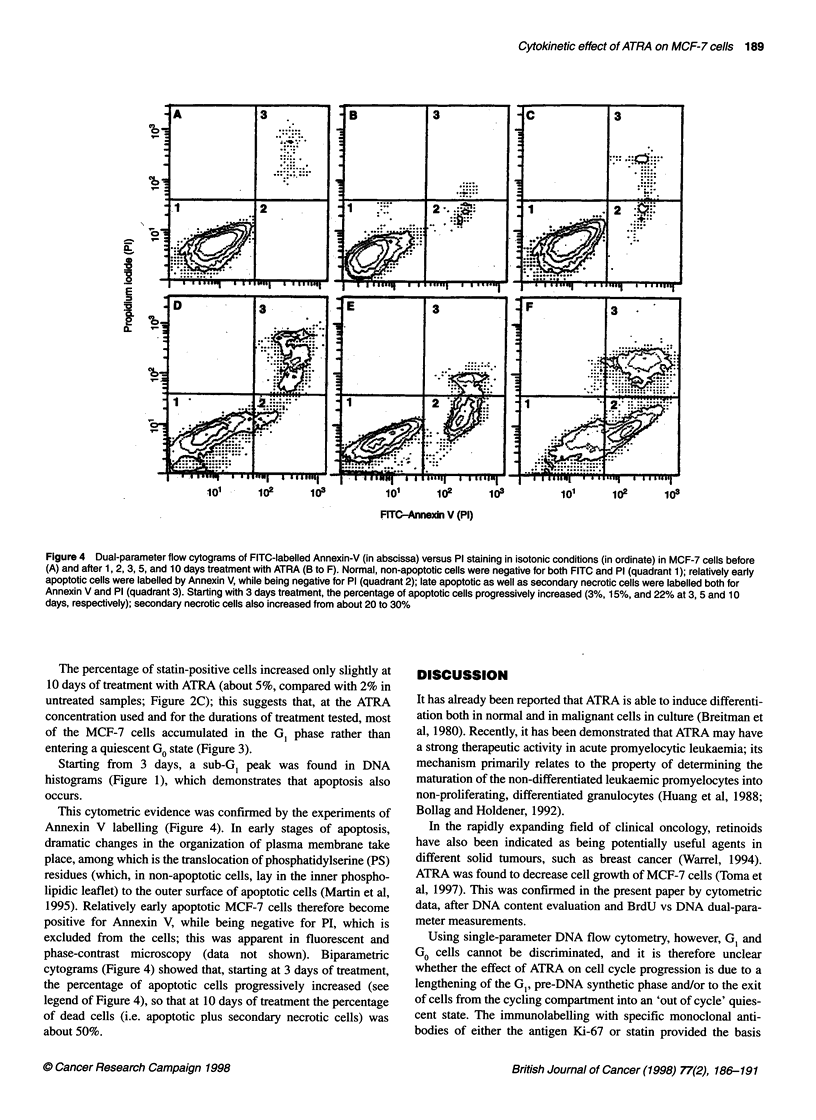
In this study the effects of all-trans retinoic acid (ATRA) on cell cycle and apoptosis of MCF-7 human breast cancer cells were investigated to elucidate the mechanisms underlying the antineoplastic potential of this retinoid in breast cancer. The antiproliferative effect of ATRA was evaluated by DNA content measurements and dual-parameter flow cytometry of bromodeoxyuridine (BrdU) incorporation and of the expression of cell cycle-related proteins (Ki-67 as proliferation marker and statin as quiescence marker) vs DNA content. Apoptosis was also studied by flow cytometry of either DNA content or Annexin V labelling. After 10(-6) M ATRA treatment, the fraction of S-phase cells decreased significantly, and cells accumulated in the G0/G1 range of DNA contents. Dual-parameter flow cytograms showed a decrease in the percentage of Ki-67-labelled cells (after 10 days, only 20% of the cells were still positive for Ki-67 compared with 95% in controls), while the fraction of statin-positive cells increased slightly. From 3 days of treatment onwards, apoptosis was found to occur. These results show that ATRA-induced inhibition of MCF-7 cell growth is related to two mechanisms, i.e. the block of cell proliferation, mostly in a pre-S phase, and the induction of apoptosis. These results should be taken into account when attempting to design treatment programmes that associate ATRA with antineoplastic compounds of different cell cycle specificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila-Cariño J., Lewin N., Tomita Y., Szeles A., Sandlund A., Mosolits S., Mellstedt H., Klein G., Klein E. B-CLL cells with unusual properties. Int J Cancer. 1997 Jan 6;70(1):1–8. doi: 10.1002/(sici)1097-0215(19970106)70:1<1::aid-ijc1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Bollag W., Holdener E. E. Retinoids in cancer prevention and therapy. Ann Oncol. 1992 Jul;3(7):513–526. doi: 10.1093/oxfordjournals.annonc.a058252. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S., Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992 Jan;25(1):31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- Connor M. J. Retinoid stimulation of epidermal differentiation in vivo. Life Sci. 1986 May 19;38(20):1807–1812. doi: 10.1016/0024-3205(86)90134-7. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J. A. Interaction of retinoids and tamoxifen on the inhibition of human mammary carcinoma cell proliferation. Exp Cell Biol. 1987;55(3):136–144. doi: 10.1159/000163409. [DOI] [PubMed] [Google Scholar]
- Fontana J. A., Miksis G., Miranda D. M., Durham J. P. Inhibition of human mammary carcinoma cell proliferation by retinoids and intracellular cAMP-elevating compounds. J Natl Cancer Inst. 1987 Jun;78(6):1107–1112. [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Huang M. E., Ye Y. C., Chen S. R., Chai J. R., Lu J. X., Zhoa L., Gu L. J., Wang Z. Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988 Aug;72(2):567–572. [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman S. M., Parkinson D. R., Itri L. M., Weber R. S., Schantz S. P., Ota D. M., Schusterman M. A., Krakoff I. H., Gutterman J. U., Hong W. K. 13-cis-retinoic acid and interferon alpha-2a: effective combination therapy for advanced squamous cell carcinoma of the skin. J Natl Cancer Inst. 1992 Feb 19;84(4):235–241. doi: 10.1093/jnci/84.4.235. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lee M. O., Wang H. G., Li Y., Hashimoto Y., Klaus M., Reed J. C., Zhang X. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996 Mar;16(3):1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitmaker B., Bayer I., Baytner S., Gordon P. H., Wang E. The differential expression of statin in the nuclei of human colonic crypts adjacent to a cancer: an immunohistochemical study. Eur J Histochem. 1993;37(1):43–51. [PubMed] [Google Scholar]
- Pellicciari C., Bottone M. G., Schaack V., Barni S., Manfredi A. A. Spontaneous apoptosis of thymocytes is uncoupled with progression through the cell cycle. Exp Cell Res. 1996 Dec 15;229(2):370–377. doi: 10.1006/excr.1996.0382. [DOI] [PubMed] [Google Scholar]
- Pellicciari C., Danova M., Giordano M., Fuhrman Conti A. M., Mazzini G., Wang E., Ronchetti E., Riccardi A., Manfredi Romanini M. G. Expression of cell cycle related proteins--proliferating cell nuclear antigen (PCNA) and statin--during adaptation and de-adaptation of EUE cells to a hypertonic medium. Cell Prolif. 1991 Sep;24(5):469–479. doi: 10.1111/j.1365-2184.1991.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Pellicciari C., Manfredi A. A., Bottone M. G., Schaack V., Barni S. A single-step staining procedure for the detection and sorting of unfixed apoptotic thymocytes. Eur J Histochem. 1993;37(4):381–390. [PubMed] [Google Scholar]
- Pellicciari C., Mangiarotti R., Bottone M. G., Danova M., Wang E. Identification of resting cells by dual-parameter flow cytometry of statin expression and DNA content. Cytometry. 1995 Dec 1;21(4):329–337. doi: 10.1002/cyto.990210404. [DOI] [PubMed] [Google Scholar]
- Schlüter C., Duchrow M., Wohlenberg C., Becker M. H., Key G., Flad H. D., Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993 Nov;123(3):513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. A 57,000-mol-wt protein uniquely present in nonproliferating cells and senescent human fibroblasts. J Cell Biol. 1985 Feb;100(2):545–551. doi: 10.1083/jcb.100.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Krueger J. G. Application of a unique monoclonal antibody as a marker for nonproliferating subpopulations of cells of some tissue. J Histochem Cytochem. 1985 Jun;33(6):587–594. doi: 10.1177/33.6.3889143. [DOI] [PubMed] [Google Scholar]
- Wilcken N. R., Sarcevic B., Musgrove E. A., Sutherland R. L. Differential effects of retinoids and antiestrogens on cell cycle progression and cell cycle regulatory genes in human breast cancer cells. Cell Growth Differ. 1996 Jan;7(1):65–74. [PubMed] [Google Scholar]


