Abstract
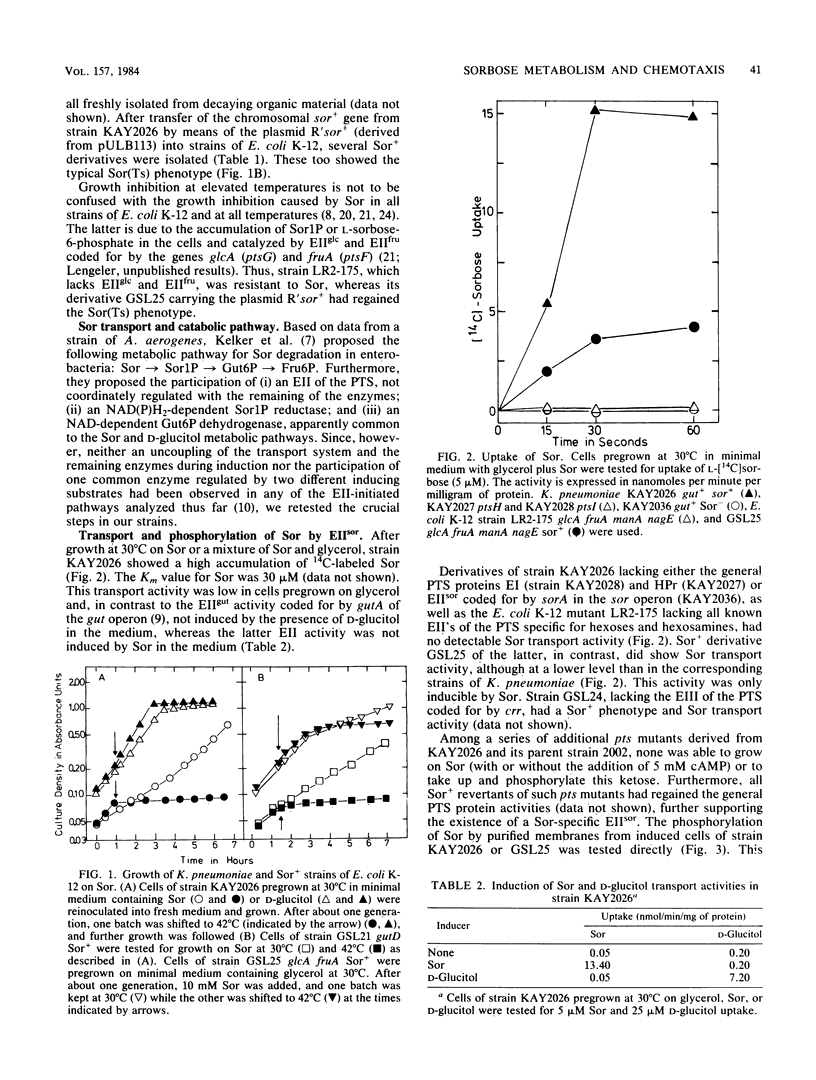
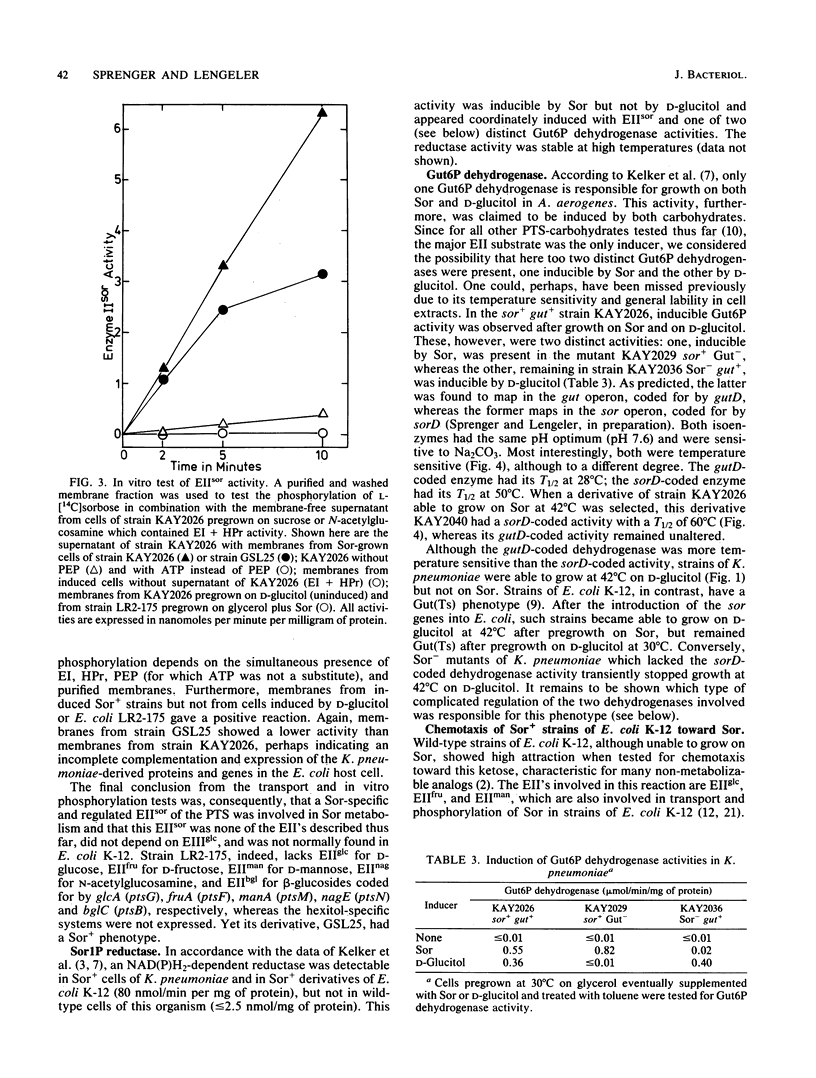
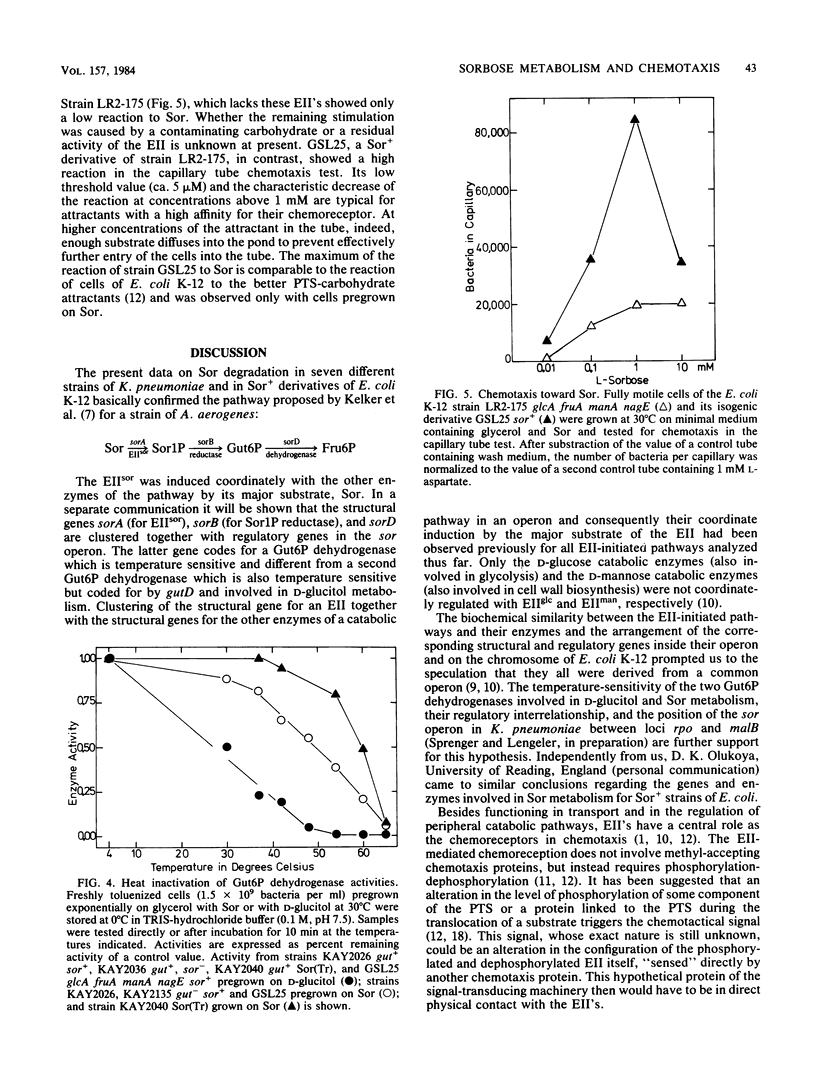
L-Sorbose degradation in Klebsiella pneumoniae was shown to follow the pathway L-sorbose leads to L-sorbose-1-phosphate leads to D-glucitol-6-phosphate leads to D-fructose-6-phosphate. Transport and phosphorylation of L-sorbose was catalyzed by membrane-bound enzyme IIsor of the phosphoenolpyruvate-dependent carbohydrate:phosphotransferase system, specific for and regulated by this ketose and different from all other enzymes II described thus far. Two soluble enzymes, an L-sorbose-1-phosphate reductase and a D-glucitol-6-phosphate dehydrogenase, were involved in the conversion of L-sorbose-1-phosphate to D-fructose-6-phosphate. This dehydrogenase was temperature sensitive, preventing growth of wild-type strains of K. pneumoniae at temperatures above 35 degrees C in the presence of L-sorbose. The enzyme was distinct from a second D-glucitol-6-phosphate dehydrogenase involved in the metabolism of D-glucitol. The sor genes were transferred from the chromosome of nonmotile strains of K. pneumoniae by means of a new R'sor+ plasmid to motile strains of Escherichia coli K-12. Such derivatives not only showed the temperature-sensitive Sor+ phenotype characteristic for K. pneumoniae or Sor+ wild-type strains of E. coli, but also reacted positively to sorbose in chemotaxis tests.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. L., Simkins R. A. L-Sorbose-1-phosphate reductase. Methods Enzymol. 1982;89(Pt 500):248–251. doi: 10.1016/s0076-6879(82)89044-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Adenylate cyclase is required for chemotaxis to phosphotransferase system sugars by Escherichia coli. J Bacteriol. 1983 Mar;153(3):1187–1195. doi: 10.1128/jb.153.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolution of ribosomal proteins in Enterobacteriaceae. J Bacteriol. 1978 Mar;133(3):1089–1095. doi: 10.1128/jb.133.3.1089-1095.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. A NEW METHOD FOR THE SELECTION OF MUTANTS OF ESCHERICHIA COLI FORMING BETA-GALACTOSIDASE CONSTITUTIVELY. Biochim Biophys Acta. 1964 Sep 4;90:609–610. doi: 10.1016/0304-4165(64)90241-7. [DOI] [PubMed] [Google Scholar]
- Kelker N. E., Simkins R. A., Anderson R. L. Pathway of L-sorbose metabolism in Aerobacter aerogenes. J Biol Chem. 1972 Mar 10;247(5):1479–1483. [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Blumenberg M., Yanofsky C. Comparison of the nucleoside sequence of trpA and sequences immediately beyond the trp operon of Klebsiella aerogenes. Salmonella typhimurium and Escherichia coli. Nucleic Acids Res. 1981 Apr 10;9(7):1743–1755. doi: 10.1093/nar/9.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A. C., Jones-Mortimer M. C., Kornberg H. L. L-Sorbose phosphorylation in Escherichia coli K-12. Biochim Biophys Acta. 1981 Aug 20;646(2):365–367. doi: 10.1016/0005-2736(81)90346-1. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Woodward M. J., Charles H. P. Genes for l-sorbose utilization in Escherichia coli. J Gen Microbiol. 1982 Sep;128(9):1969–1980. doi: 10.1099/00221287-128-9-1969. [DOI] [PubMed] [Google Scholar]


