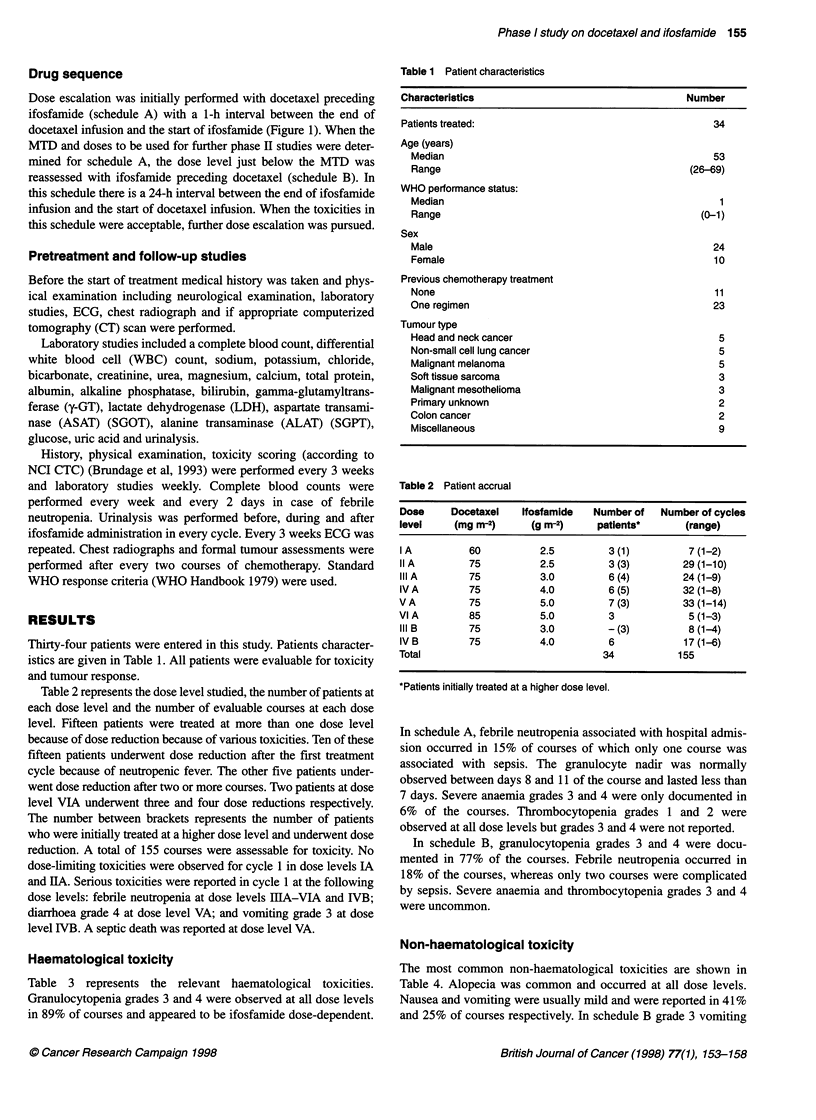
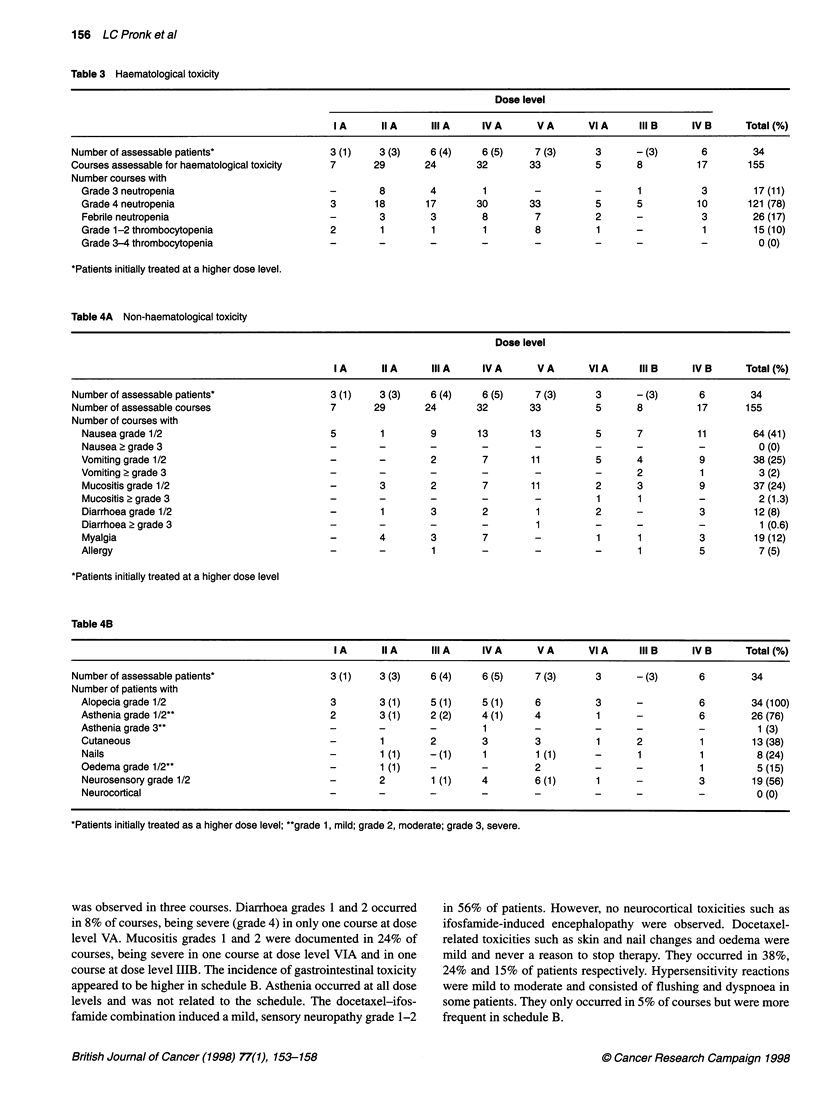
Abstract
Docetaxel and ifosfamide have shown significant activity against a variety of solid tumours. This prompted a phase I trial on the combination of these drugs. This phase I study was performed to assess the feasibility of the combination, to determine the maximum tolerated dose (MTD) and the side effects, and to propose a safe schedule for further phase II studies. A total of 34 patients with a histologically confirmed solid tumour, who were not pretreated with taxanes or ifosfamide and who had received no more than one line of chemotherapy for advanced disease were entered into the study. Treatment consisted of docetaxel given as a 1-h infusion followed by ifosfamide as a 24-h infusion (schedule A), or ifosfamide followed by docetaxel (schedule B) every 3 weeks. Docetaxel doses ranged from 60 to 85 mg m(-2) and ifosfamide doses from 2.5 to 5.0 g m(-2). Granulocytopenia grade 3 and 4 were common (89%), short lasting and ifosfamide dose dependent. Febrile neutropenia and sepsis occurred in 17% and 2% of courses respectively. Non-haematological toxicities were mild to moderate and included alopecia, nausea, vomiting, mucositis, diarrhoea, sensory neuropathy, skin and nail toxicity and oedema. There did not appear to be any pharmacokinetic interaction between docetaxel and ifosfamide. One complete response (CR) (soft tissue sarcoma) and two partial responses (PRs) were documented. A dose of 75 mg m(-2) of docetaxel combined with 5.0 g m(-2) ifosfamide appeared to be manageable. Schedule A was advocated for further treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamdal S., Wolff I., Kaplan S., Paridaens R., Kerger J., Schachter J., Wanders J., Franklin H. R., Verweij J. Docetaxel (Taxotere) in advanced malignant melanoma: a phase II study of the EORTC Early Clinical Trials Group. Eur J Cancer. 1994;30A(8):1061–1064. doi: 10.1016/0959-8049(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Bissery M. C., Guénard D., Guéritte-Voegelein F., Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991 Sep 15;51(18):4845–4852. [PubMed] [Google Scholar]
- Bissett D., Setanoians A., Cassidy J., Graham M. A., Chadwick G. A., Wilson P., Auzannet V., Le Bail N., Kaye S. B., Kerr D. J. Phase I and pharmacokinetic study of taxotere (RP 56976) administered as a 24-hour infusion. Cancer Res. 1993 Feb 1;53(3):523–527. [PubMed] [Google Scholar]
- Brundage M. D., Pater J. L., Zee B. Assessing the reliability of two toxicity scales: implications for interpreting toxicity data. J Natl Cancer Inst. 1993 Jul 21;85(14):1138–1148. doi: 10.1093/jnci/85.14.1138. [DOI] [PubMed] [Google Scholar]
- Burris H., Irvin R., Kuhn J., Kalter S., Smith L., Shaffer D., Fields S., Weiss G., Eckardt J., Rodriguez G. Phase I clinical trial of taxotere administered as either a 2-hour or 6-hour intravenous infusion. J Clin Oncol. 1993 May;11(5):950–958. doi: 10.1200/JCO.1993.11.5.950. [DOI] [PubMed] [Google Scholar]
- Catimel G., Verweij J., Mattijssen V., Hanauske A., Piccart M., Wanders J., Franklin H., Le Bail N., Clavel M., Kaye S. B. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994 Jul;5(6):533–537. doi: 10.1093/oxfordjournals.annonc.a058908. [DOI] [PubMed] [Google Scholar]
- Cerny T., Kaplan S., Pavlidis N., Schöffski P., Epelbaum R., van Meerbeek J., Wanders J., Franklin H. R., Kaye S. Docetaxel (Taxotere) is active in non-small-cell lung cancer: a phase II trial of the EORTC Early Clinical Trials Group (ECTG) Br J Cancer. 1994 Aug;70(2):384–387. doi: 10.1038/bjc.1994.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier B., Fumoleau P., Kerbrat P., Dieras V., Roche H., Krakowski I., Azli N., Bayssas M., Lentz M. A., Van Glabbeke M. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1995 Feb;13(2):314–322. doi: 10.1200/JCO.1995.13.2.314. [DOI] [PubMed] [Google Scholar]
- Ettinger D. S. Ifosfamide in the treatment of non-small cell lung cancer. Semin Oncol. 1989 Feb;16(1 Suppl 3):31–38. [PubMed] [Google Scholar]
- Extra J. M., Rousseau F., Bruno R., Clavel M., Le Bail N., Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993 Mar 1;53(5):1037–1042. [PubMed] [Google Scholar]
- Fossella F. V., Lee J. S., Shin D. M., Calayag M., Huber M., Perez-Soler R., Murphy W. K., Lippman S., Benner S., Glisson B. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995 Mar;13(3):645–651. doi: 10.1200/JCO.1995.13.3.645. [DOI] [PubMed] [Google Scholar]
- Guéritte-Voegelein F., Guénard D., Lavelle F., Le Goff M. T., Mangatal L., Potier P. Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem. 1991 Mar;34(3):992–998. doi: 10.1021/jm00107a017. [DOI] [PubMed] [Google Scholar]
- Hilkens P. H., Pronk L. C., Verweij J., Vecht C. J., van Putten W. L., van den Bent M. J. Peripheral neuropathy induced by combination chemotherapy of docetaxel and cisplatin. Br J Cancer. 1997;75(3):417–422. doi: 10.1038/bjc.1997.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens P. H., Verweij J., Stoter G., Vecht C. J., van Putten W. L., van den Bent M. J. Peripheral neurotoxicity induced by docetaxel. Neurology. 1996 Jan;46(1):104–108. doi: 10.1212/wnl.46.1.104. [DOI] [PubMed] [Google Scholar]
- Miller V. A., Rigas J. R., Francis P. A., Grant S. C., Pisters K. M., Venkatraman E. S., Woolley K., Heelan R. T., Kris M. G. Phase II trial of a 75-mg/m2 dose of docetaxel with prednisone premedication for patients with advanced non-small cell lung cancer. Cancer. 1995 Feb 15;75(4):968–972. doi: 10.1002/1097-0142(19950215)75:4<968::aid-cncr2820750411>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pazdur R., Newman R. A., Newman B. M., Fuentes A., Benvenuto J., Bready B., Moore D., Jr, Jaiyesimi I., Vreeland F., Bayssas M. M. Phase I trial of Taxotere: five-day schedule. J Natl Cancer Inst. 1992 Dec 2;84(23):1781–1788. doi: 10.1093/jnci/84.23.1781. [DOI] [PubMed] [Google Scholar]
- Pinedo H. M., Verweij J. The treatment of soft tissue sarcomas with focus on chemotherapy: a review. Radiother Oncol. 1986 Mar;5(3):193–205. doi: 10.1016/s0167-8140(86)80049-4. [DOI] [PubMed] [Google Scholar]
- Pronk L. C., Schellens J. H., Planting A. S., van den Bent M. J., Hilkens P. H., van der Burg M. E., de Boer-Dennert M., Ma J., Blanc C., Harteveld M. Phase I and pharmacologic study of docetaxel and cisplatin in patients with advanced solid tumors. J Clin Oncol. 1997 Mar;15(3):1071–1079. doi: 10.1200/JCO.1997.15.3.1071. [DOI] [PubMed] [Google Scholar]
- Pronk L. C., Stoter G., Verweij J. Docetaxel (Taxotere): single agent activity, development of combination treatment and reducing side-effects. Cancer Treat Rev. 1995 Sep;21(5):463–478. doi: 10.1016/0305-7372(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Ringel I., Horwitz S. B. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991 Feb 20;83(4):288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Donehower R. C. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther. 1991 Oct;52(1):35–84. doi: 10.1016/0163-7258(91)90086-2. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Gilbert M. R., McGuire W. P., Noe D. A., Grochow L. B., Forastiere A. A., Ettinger D. S., Lubejko B. G., Clark B., Sartorius S. E. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991 Sep;9(9):1692–1703. doi: 10.1200/JCO.1991.9.9.1692. [DOI] [PubMed] [Google Scholar]
- Schrijvers D., Wanders J., Dirix L., Prove A., Vonck I., van Oosterom A., Kaye S. Coping with toxicities of docetaxel (Taxotere). Ann Oncol. 1993 Aug;4(7):610–611. doi: 10.1093/oxfordjournals.annonc.a058599. [DOI] [PubMed] [Google Scholar]
- Sulkes A., Smyth J., Sessa C., Dirix L. Y., Vermorken J. B., Kaye S., Wanders J., Franklin H., LeBail N., Verweij J. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994 Aug;70(2):380–383. doi: 10.1038/bjc.1994.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiak E., Piccart M. J., Kerger J., Lips S., Awada A., de Valeriola D., Ravoet C., Lossignol D., Sculier J. P., Auzannet V. Phase I study of docetaxel administered as a 1-hour intravenous infusion on a weekly basis. J Clin Oncol. 1994 Jul;12(7):1458–1467. doi: 10.1200/JCO.1994.12.7.1458. [DOI] [PubMed] [Google Scholar]
- Wilke H., Siewert J. R., Fink U., Stahl M. Current status and future directions in the treatment of localized esophageal cancer. Ann Oncol. 1994;5 (Suppl 3):27–32. doi: 10.1093/annonc/5.suppl_3.s27. [DOI] [PubMed] [Google Scholar]
- ten Bokkel Huinink W. W., Prove A. M., Piccart M., Steward W., Tursz T., Wanders J., Franklin H., Clavel M., Verweij J., Alakl M. A phase II trial with docetaxel (Taxotere) in second line treatment with chemotherapy for advanced breast cancer. A study of the EORTC Early Clinical Trials Group. Ann Oncol. 1994 Jul;5(6):527–532. [PubMed] [Google Scholar]
- van Hoesel Q. G., Verweij J., Catimel G., Clavel M., Kerbrat P., van Oosterom A. T., Kerger J., Tursz T., van Glabbeke M., van Pottelsberghe C. Phase II study with docetaxel (Taxotere) in advanced soft tissue sarcomas of the adult. EORTC Soft Tissue and Bone Sarcoma Group. Ann Oncol. 1994 Jul;5(6):539–542. doi: 10.1093/oxfordjournals.annonc.a058909. [DOI] [PubMed] [Google Scholar]


