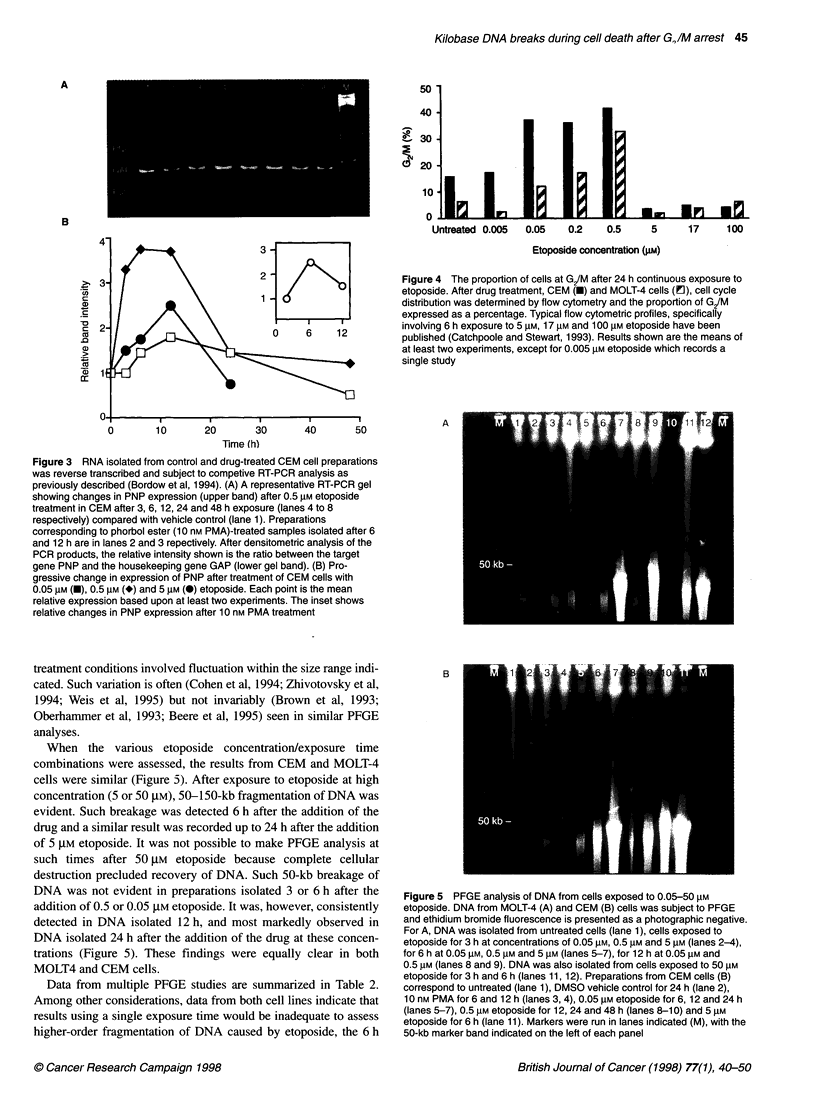
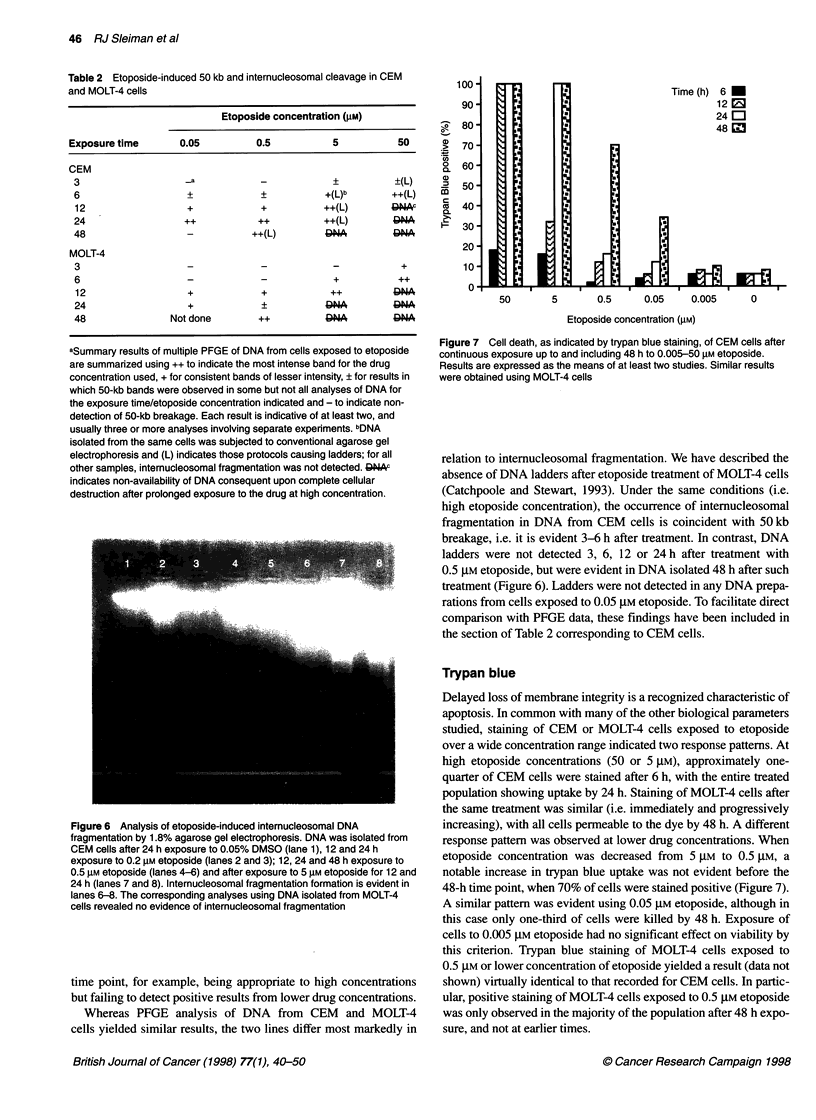
Abstract
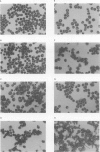
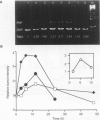
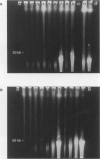
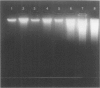
Many reports have documented apoptotic death in different cell types within hours of exposure to cytotoxic drugs; lower drug concentrations may cause cell cycle arrest at G2/M and subsequent death, which has been distinguished from 'classic' apoptosis. We have analysed etoposide-induced cell death in two lymphoblastoid T-cell lines, CCRF-CEM and MOLT-4, specifically in relation to DNA cleavage as indicated by pulse-field gel and conventional electrophoresis. High (5 microM) concentration etoposide causes 50-kb cleavage of DNA that occurs at the same time as apoptotic morphology and internucleosomal cleavage. At lower concentrations (0.5-0.05 microM), sequential change may be discerned with altered gene expression being similar to that at high dose, but preceding cell cycle arrest and 50-kb cleavage. These last changes, in turn, clearly precede internucleosomal fragmentation of DNA, vital dye staining and morphological evidence cell death. The pattern of higher order fragmentation constitutes a sensitive indicator of commitment to cell death in these cells. Morphological evidence of cell death is associated with internucleosomal fragmentation in one of the lines, but the pattern of 50-kb DNA cleavage provides the clearest evidence of commonality in death processes occurring at low and high drug concentration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. D., Bustin S. A., Newland A. C. The role of apoptosis (programmed cell death) in haemopoiesis and the immune system. Blood Rev. 1993 Mar;7(1):63–73. doi: 10.1016/0268-960x(93)90025-y. [DOI] [PubMed] [Google Scholar]
- An B., Dou Q. P. Cleavage of retinoblastoma protein during apoptosis: an interleukin 1 beta-converting enzyme-like protease as candidate. Cancer Res. 1996 Feb 1;56(3):438–442. [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem Biophys Res Commun. 1992 Jul 31;186(2):782–789. doi: 10.1016/0006-291x(92)90814-2. [DOI] [PubMed] [Google Scholar]
- Beere H. M., Chresta C. M., Alejo-Herberg A., Skladanowski A., Dive C., Larsen A. K., Hickman J. A. Investigation of the mechanism of higher order chromatin fragmentation observed in drug-induced apoptosis. Mol Pharmacol. 1995 May;47(5):986–996. [PubMed] [Google Scholar]
- Bertrand R., Sarang M., Jenkin J., Kerrigan D., Pommier Y. Differential induction of secondary DNA fragmentation by topoisomerase II inhibitors in human tumor cell lines with amplified c-myc expression. Cancer Res. 1991 Dec 1;51(23 Pt 1):6280–6285. [PubMed] [Google Scholar]
- Bonelli G., Sacchi M. C., Barbiero G., Duranti F., Goglio G., Verdun di Cantogno L., Amenta J. S., Piacentini M., Tacchetti C., Baccino F. M. Apoptosis of L929 cells by etoposide: a quantitative and kinetic approach. Exp Cell Res. 1996 Nov 1;228(2):292–305. doi: 10.1006/excr.1996.0329. [DOI] [PubMed] [Google Scholar]
- Bordow S. B., Haber M., Madafiglio J., Cheung B., Marshall G. M., Norris M. D. Expression of the multidrug resistance-associated protein (MRP) gene correlates with amplification and overexpression of the N-myc oncogene in childhood neuroblastoma. Cancer Res. 1994 Oct 1;54(19):5036–5040. [PubMed] [Google Scholar]
- Bortner C. D., Oldenburg N. B., Cidlowski J. A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995 Jan;5(1):21–26. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- Brown D. G., Sun X. M., Cohen G. M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem. 1993 Feb 15;268(5):3037–3039. [PubMed] [Google Scholar]
- Campana D., Janossy G. Proliferation of normal and malignant human immature lymphoid cells. Blood. 1988 May;71(5):1201–1210. [PubMed] [Google Scholar]
- Catchpoole D. R., Stewart B. W. Etoposide-induced cytotoxicity in two human T-cell leukemic lines: delayed loss of membrane permeability rather than DNA fragmentation as an indicator of programmed cell death. Cancer Res. 1993 Sep 15;53(18):4287–4296. [PubMed] [Google Scholar]
- Catchpoole D. R., Stewart B. W. Formation of apoptotic bodies is associated with internucleosomal DNA fragmentation during drug-induced apoptosis. Exp Cell Res. 1995 Jan;216(1):169–177. doi: 10.1006/excr.1995.1021. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991 Sep;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Chen I. T., Smith M. L., O'Connor P. M., Fornace A. J., Jr Direct interaction of Gadd45 with PCNA and evidence for competitive interaction of Gadd45 and p21Waf1/Cip1 with PCNA. Oncogene. 1995 Nov 16;11(10):1931–1937. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chow K. C., Ross W. E. Topoisomerase-specific drug sensitivity in relation to cell cycle progression. Mol Cell Biol. 1987 Sep;7(9):3119–3123. doi: 10.1128/mcb.7.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Fearnhead H., MacFarlane M., Brown D. G., Snowden R. T., Dinsdale D. Formation of large molecular weight fragments of DNA is a key committed step of apoptosis in thymocytes. J Immunol. 1994 Jul 15;153(2):507–516. [PubMed] [Google Scholar]
- Compton M. M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992 Sep;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Constantinou A., Grdina D., Kiguchi K., Huberman E. The effect of topoisomerase inhibitors on the expression of differentiation markers and cell cycle progression in human K-562 leukemia cells. Exp Cell Res. 1992 Nov;203(1):100–106. doi: 10.1016/0014-4827(92)90044-9. [DOI] [PubMed] [Google Scholar]
- Deitch A. D., Law H., deVere White R. A stable propidium iodide staining procedure for flow cytometry. J Histochem Cytochem. 1982 Sep;30(9):967–972. doi: 10.1177/30.9.6182188. [DOI] [PubMed] [Google Scholar]
- Del Bino G., Skierski J. S., Darzynkiewicz Z. The concentration-dependent diversity of effects of DNA topoisomerase I and II inhibitors on the cell cycle of HL-60 cells. Exp Cell Res. 1991 Aug;195(2):485–491. doi: 10.1016/0014-4827(91)90400-o. [DOI] [PubMed] [Google Scholar]
- Demarcq C., Bunch R. T., Creswell D., Eastman A. The role of cell cycle progression in cisplatin-induced apoptosis in Chinese hamster ovary cells. Cell Growth Differ. 1994 Sep;5(9):983–993. [PubMed] [Google Scholar]
- Desjardins L. M., MacManus J. P. An adherent cell model to study different stages of apoptosis. Exp Cell Res. 1995 Feb;216(2):380–387. doi: 10.1006/excr.1995.1048. [DOI] [PubMed] [Google Scholar]
- Dou Q. P., An B., Will P. L. Induction of a retinoblastoma phosphatase activity by anticancer drugs accompanies p53-independent G1 arrest and apoptosis. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9019–9023. doi: 10.1073/pnas.92.20.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos A. G., Kerr D. J., Herod J., Hodgkins L., Krajewski S., Reed J. C., Young L. S. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995 Oct 5;11(7):1217–1228. [PubMed] [Google Scholar]
- Falcieri E., Martelli A. M., Bareggi R., Cataldi A., Cocco L. The protein kinase inhibitor staurosporine induces morphological changes typical of apoptosis in MOLT-4 cells without concomitant DNA fragmentation. Biochem Biophys Res Commun. 1993 May 28;193(1):19–25. doi: 10.1006/bbrc.1993.1584. [DOI] [PubMed] [Google Scholar]
- Filipski J., Leblanc J., Youdale T., Sikorska M., Walker P. R. Periodicity of DNA folding in higher order chromatin structures. EMBO J. 1990 Apr;9(4):1319–1327. doi: 10.1002/j.1460-2075.1990.tb08241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S., Freemerman A. J., Birrer M. J., Martin H. A., Turner A. J., Szabo E., Chelliah J., Jarvis W. D. Effect of 1-beta-D-arabinofuranosylcytosine on apoptosis and differentiation in human monocytic leukemia cells (U937) expressing a c-Jun dominant-negative mutant protein (TAM67). Cell Growth Differ. 1996 May;7(5):603–613. [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Hotz M. A., Del Bino G., Lassota P., Traganos F., Darzynkiewicz Z. Cytostatic and cytotoxic effects of fostriecin on human promyelocytic HL-60 and lymphocytic MOLT-4 leukemic cells. Cancer Res. 1992 Mar 15;52(6):1530–1535. [PubMed] [Google Scholar]
- Jordan M. A., Wendell K., Gardiner S., Derry W. B., Copp H., Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996 Feb 15;56(4):816–825. [PubMed] [Google Scholar]
- Lazebnik Y. A., Takahashi A., Moir R. D., Goldman R. D., Poirier G. G., Kaufmann S. H., Earnshaw W. C. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Li C. J., Wang C., Pardee A. B. Induction of apoptosis by beta-lapachone in human prostate cancer cells. Cancer Res. 1995 Sep 1;55(17):3712–3715. [PubMed] [Google Scholar]
- Lock R. B., Galperina O. V., Feldhoff R. C., Rhodes L. J. Concentration-dependent differences in the mechanisms by which caffeine potentiates etoposide cytotoxicity in HeLa cells. Cancer Res. 1994 Sep 15;54(18):4933–4939. [PubMed] [Google Scholar]
- Lock R. B., Stribinskiene L. Dual modes of death induced by etoposide in human epithelial tumor cells allow Bcl-2 to inhibit apoptosis without affecting clonogenic survival. Cancer Res. 1996 Sep 1;56(17):4006–4012. [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Madrid-Marina V., Martinez-Valdez H., Cohen A. Phorbol esters induce changes in adenosine deaminase, purine nucleoside phosphorylase, and terminal deoxynucleotidyl transferase messenger RNA levels in human leukemic cell lines. Cancer Res. 1990 May 15;50(10):2891–2894. [PubMed] [Google Scholar]
- Marks D. I., Fox R. M. DNA damage, poly (ADP-ribosyl)ation and apoptotic cell death as a potential common pathway of cytotoxic drug action. Biochem Pharmacol. 1991 Oct 24;42(10):1859–1867. doi: 10.1016/0006-2952(91)90582-p. [DOI] [PubMed] [Google Scholar]
- Marks D. I., Kurz B. W., Link M. P., Ng E., Shuster J. J., Lauer S. J., Brodsky I., Haines D. S. High incidence of potential p53 inactivation in poor outcome childhood acute lymphoblastic leukemia at diagnosis. Blood. 1996 Feb 1;87(3):1155–1161. [PubMed] [Google Scholar]
- Martinez-Valdez H., Cohen A. Coordinate regulation of mRNAs encoding adenosine deaminase, purine nucleoside phosphorylase, and terminal deoxynucleotidyltransferase by phorbol esters in human thymocytes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6900–6903. doi: 10.1073/pnas.85.18.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Sweeney E. A., Masamune A., Yatomi Y., Hakomori S., Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995 Feb 1;55(3):691–697. [PubMed] [Google Scholar]
- Ormerod M. G., Orr R. M., Peacock J. H. The role of apoptosis in cell killing by cisplatin: a flow cytometric study. Br J Cancer. 1994 Jan;69(1):93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. P., Rao P. N. The cause of G2-arrest in Chinese hamster ovary cells treated with anticancer drugs. J Natl Cancer Inst. 1976 Nov;57(5):1139–1143. doi: 10.1093/jnci/57.5.1139. [DOI] [PubMed] [Google Scholar]
- Rusch V., Klimstra D., Venkatraman E., Oliver J., Martini N., Gralla R., Kris M., Dmitrovsky E. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995 Nov 1;55(21):5038–5042. [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Salmon M., Pilling D., Borthwick N. J., Viner N., Janossy G., Bacon P. A., Akbar A. N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994 Apr;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Barnett S. K., Atillasoy E. S., Milstone L. M. Methotrexate induces differentiation of human keratinocytes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):594–598. doi: 10.1073/pnas.89.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. K., Duch D. S., Dev I. K., Kaufmann S. H. Metabolic effects and kill of human T-cell leukemia by 5-deazaacyclotetrahydrofolate, a specific inhibitor of glycineamide ribonucleotide transformylase. Cancer Res. 1992 Sep 15;52(18):4895–4903. [PubMed] [Google Scholar]
- Sorenson C. M., Barry M. A., Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990 May 2;82(9):749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- Stewart B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994 Sep 7;86(17):1286–1296. doi: 10.1093/jnci/86.17.1286. [DOI] [PubMed] [Google Scholar]
- Sun X. M., Cohen G. M. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J Biol Chem. 1994 May 27;269(21):14857–14860. [PubMed] [Google Scholar]
- Tornaletti S., Pfeifer G. P. Complete and tissue-independent methylation of CpG sites in the p53 gene: implications for mutations in human cancers. Oncogene. 1995 Apr 20;10(8):1493–1499. [PubMed] [Google Scholar]
- Tounekti O., Pron G., Belehradek J., Jr, Mir L. M. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993 Nov 15;53(22):5462–5469. [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. R., Weaver V. M., Lach B., LeBlanc J., Sikorska M. Endonuclease activities associated with high molecular weight and internucleosomal DNA fragmentation in apoptosis. Exp Cell Res. 1994 Jul;213(1):100–106. doi: 10.1006/excr.1994.1178. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Kanbe K., Shinozaki T., Hoshino H., Chigira M. Apoptosis of a fibrosarcoma induced by protein-free culture involves DNA cleavage to large fragments but not internucleosomal fragmentation. Int J Cancer. 1995 Jul 17;62(2):191–198. doi: 10.1002/ijc.2910620214. [DOI] [PubMed] [Google Scholar]
- Weis M., Schlegel J., Kass G. E., Holmström T. H., Peters I., Eriksson J., Orrenius S., Chow S. C. Cellular events in Fas/APO-1-mediated apoptosis in JURKAT T lymphocytes. Exp Cell Res. 1995 Aug;219(2):699–708. doi: 10.1006/excr.1995.1281. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis: cell death in tissue regulation. J Pathol. 1987 Dec;153(4):313–316. doi: 10.1002/path.1711530404. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Wade D., Gahm A., Orrenius S., Nicotera P. Formation of 50 kbp chromatin fragments in isolated liver nuclei is mediated by protease and endonuclease activation. FEBS Lett. 1994 Sep 5;351(2):150–154. doi: 10.1016/0014-5793(94)00827-2. [DOI] [PubMed] [Google Scholar]
- da Silva C. P., de Oliveira C. R., da Conceiço M., de Lima P. Apoptosis as a mechanism of cell death induced by different chemotherapeutic drugs in human leukemic T-lymphocytes. Biochem Pharmacol. 1996 May 17;51(10):1331–1340. doi: 10.1016/0006-2952(96)00041-x. [DOI] [PubMed] [Google Scholar]