Abstract
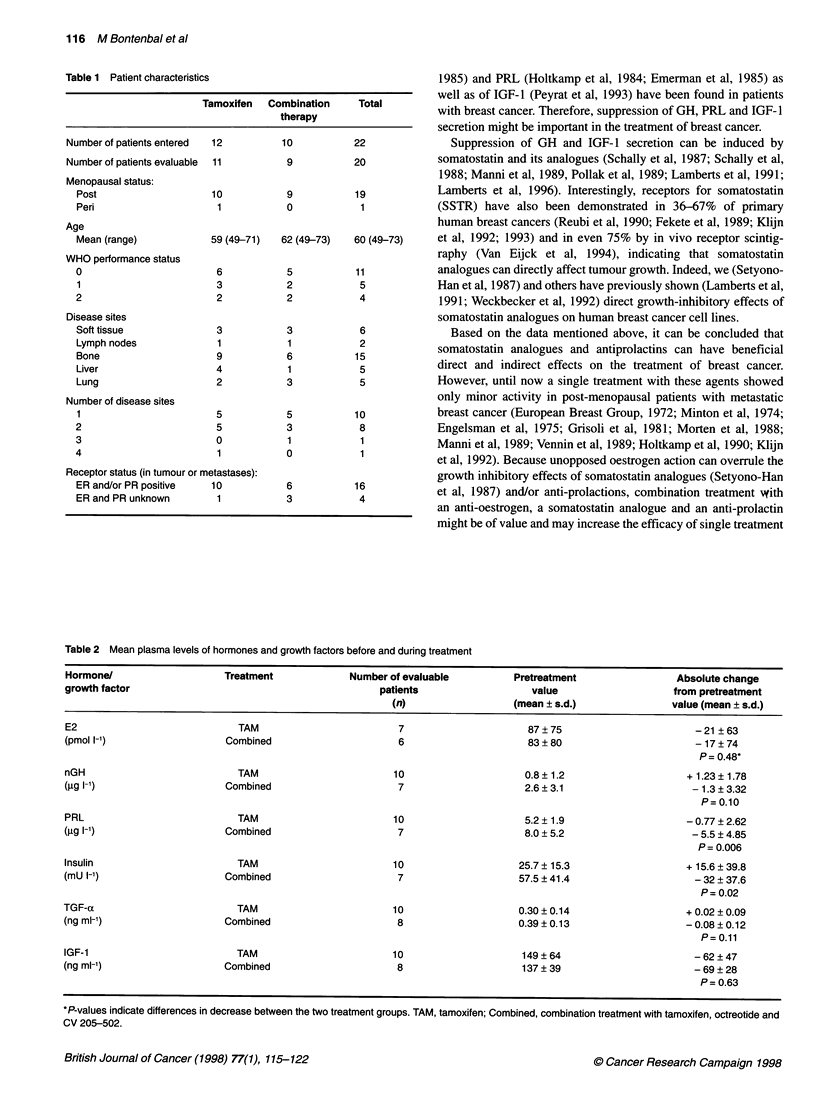
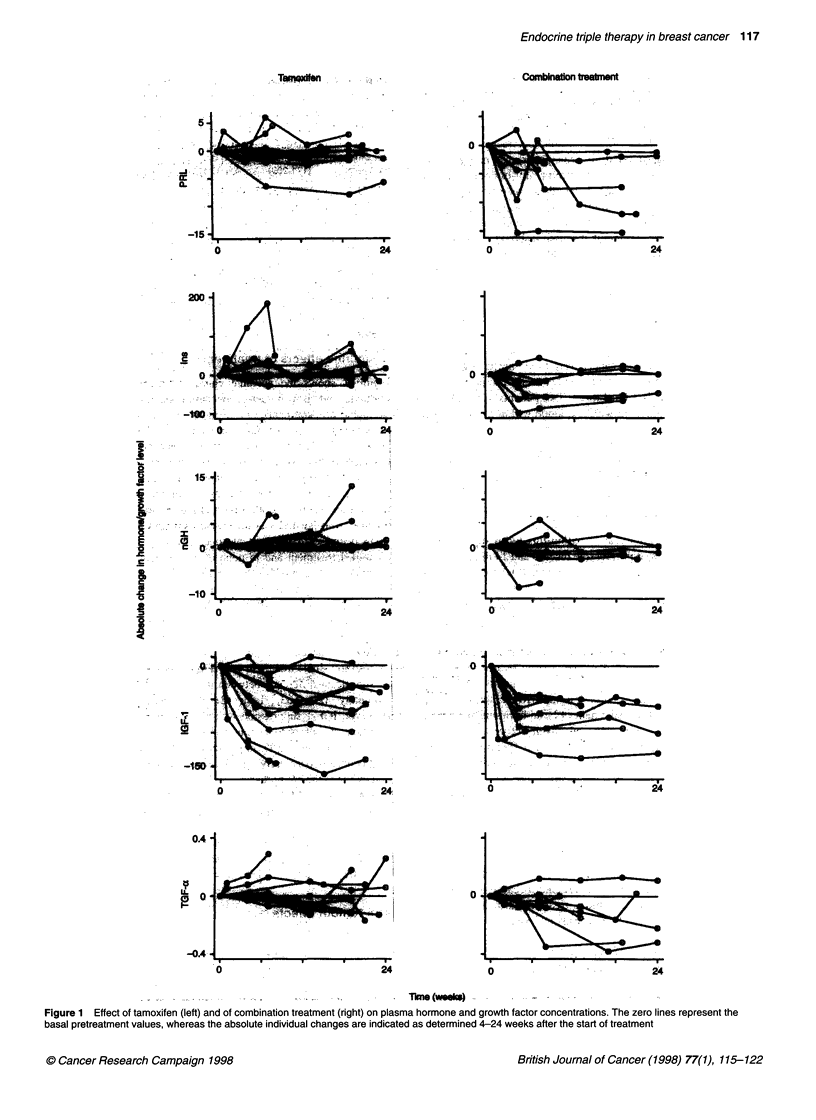
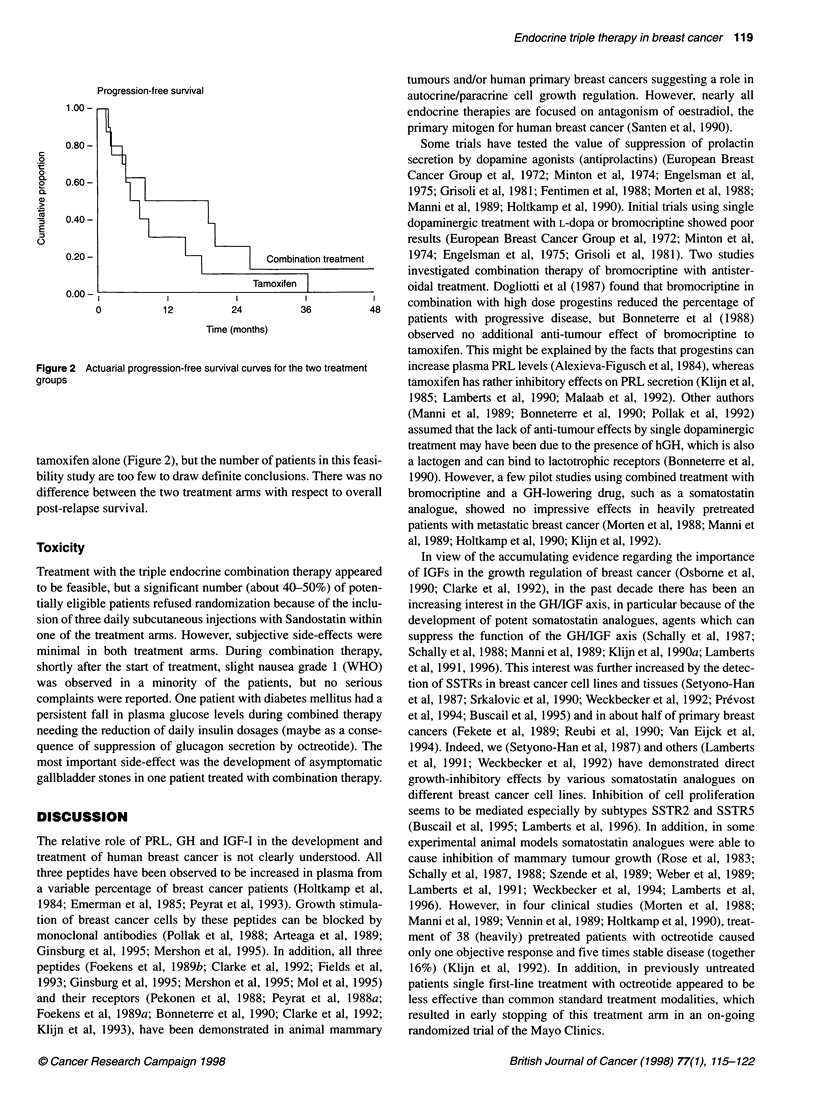
Suppression of the secretion of prolactin, growth hormone and insulin-like growth factor 1 (IGF-1) might be important in the growth regulation and treatment of breast cancer. Because oestrogens may counteract the anti-tumour effects of such treatment, the combination of an anti-oestrogen (tamoxifen), a somatostatin analogue (octreotide) and a potent anti-prolactin (CV 205-502) might be attractive. In this respect, we performed a first exploratory long-term study on the feasibility of combined treatment and possible clear differences in endocrine and anti-tumour effects during such combined treatment vs standard treatment with tamoxifen alone. Twenty-two post-menopausal patients with metastatic breast cancer (ER and/or PR positive or unknown) were randomized to receive either 40 mg of tamoxifen per day or the combination of 40 mg of tamoxifen plus 75 microg of CV 205-502 orally plus 3 x 0.2 mg of octreotide s.c. as first-line endocrine therapy. An objective response was found in 36% of the patients treated with tamoxifen alone and in 55% of the patients treated with combination therapy. Median time to progression was 33 weeks for patients treated with tamoxifen and 84 weeks for patients treated with combination therapy, but the numbers are too small for hard conclusions. There was no difference in overall post-relapse survival between the two treatment arms. With respect to the endocrine parameters, there was a significant decrease of plasma IGF-1 levels in both treatment arms, whereas during combined treatment plasma growth hormone tended to decrease and plasma prolactin levels were strongly suppressed; in some patients insulin and transforming growth factor alpha (TGF-alpha) decreased during the triple therapy. Although there was no significant difference in mean decrease of plasma IGF-1 levels between the two treatment arms, combined treatment resulted in a more uniform suppression of IGF-1. Therefore, the addition of a somatostatin analogue and an anti-prolactin may potentially enhance the efficacy of anti-oestrogens in the treatment of breast cancer owing to favourable endocrine and possible direct anti-tumour effects. Large phase III trials using depot formulations (to increase the feasibility) of somatostatin analogues are warranted to demonstrate the potential extra beneficial anti-tumour effects of such combination therapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexieva-Figusch J., Blankenstein M. A., Hop W. C., Klijn J. G., Lamberts S. W., de Jong F. H., Docter R., Adlercreutz H., van Gilse H. A. Treatment of metastatic breast cancer patients with different dosages of megestrol acetate; dose relations, metabolic and endocrine effects. Eur J Cancer Clin Oncol. 1984 Jan;20(1):33–40. doi: 10.1016/0277-5379(84)90031-2. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Osborne C. K. Growth inhibition of human breast cancer cells in vitro with an antibody against the type I somatomedin receptor. Cancer Res. 1989 Nov 15;49(22):6237–6241. [PubMed] [Google Scholar]
- Bonneterre J., Mauriac L., Weber B., Roche H., Fargeot P., Tubiana-Hulin M., Sevin M., Chollet P., Cappelaere P. Tamoxifen plus bromocriptine versus tamoxifen plus placebo in advanced breast cancer: results of a double blind multicentre clinical trial. Eur J Cancer Clin Oncol. 1988 Dec;24(12):1851–1853. doi: 10.1016/0277-5379(88)90097-1. [DOI] [PubMed] [Google Scholar]
- Bonneterre J., Peyrat J. P., Beuscart R., Demaille A. Biological and clinical aspects of prolactin receptors (PRL-R) in human breast cancer. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):977–981. doi: 10.1016/0960-0760(90)90453-r. [DOI] [PubMed] [Google Scholar]
- Buscail L., Estève J. P., Saint-Laurent N., Bertrand V., Reisine T., O'Carroll A. M., Bell G. I., Schally A. V., Vaysse N., Susini C. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1580–1584. doi: 10.1073/pnas.92.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butta A., MacLennan K., Flanders K. C., Sacks N. P., Smith I., McKinna A., Dowsett M., Wakefield L. M., Sporn M. B., Baum M. Induction of transforming growth factor beta 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992 Aug 1;52(15):4261–4264. [PubMed] [Google Scholar]
- Chiodera P., Volpi R., d'Amato L., Fatone M., Cigarini C., Fava A., Caiazza A., Rossi G., Coiro V. Inhibition by somatostatin of LH-RH-induced LH release in normal menstruating women. Gynecol Obstet Invest. 1986;22(1):17–21. doi: 10.1159/000298884. [DOI] [PubMed] [Google Scholar]
- Clarke R., Dickson R. B., Lippman M. E. Hormonal aspects of breast cancer. Growth factors, drugs and stromal interactions. Crit Rev Oncol Hematol. 1992 Jan;12(1):1–23. doi: 10.1016/1040-8428(92)90062-u. [DOI] [PubMed] [Google Scholar]
- Colletti R. B., Roberts J. D., Devlin J. T., Copeland K. C. Effect of tamoxifen on plasma insulin-like growth factor I in patients with breast cancer. Cancer Res. 1989 Apr 1;49(7):1882–1884. [PubMed] [Google Scholar]
- Cullen K. J., Lippman M. E., Chow D., Hill S., Rosen N., Zwiebel J. A. Insulin-like growth factor-II overexpression in MCF-7 cells induces phenotypic changes associated with malignant progression. Mol Endocrinol. 1992 Jan;6(1):91–100. doi: 10.1210/mend.6.1.1310798. [DOI] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Growth hormone increases ovarian levels of immunoreactive somatomedin C/insulin-like growth factor I in vivo. Endocrinology. 1986 Feb;118(2):888–890. doi: 10.1210/endo-118-2-888. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Leahy M., Gout P. W., Bruchovsky N. Elevated growth hormone levels in sera from breast cancer patients. Horm Metab Res. 1985 Aug;17(8):421–424. doi: 10.1055/s-2007-1013563. [DOI] [PubMed] [Google Scholar]
- Engelsman E., Heuson J. C., Blonk Van Der Wijst J., Drochmans A., Maass H., Cheix F., Sobrinho L. G., Nowakowski H. Controlled clinical trial of L-dopa and nafoxidine in advanced breast cancer: an E.O.R.T.C. study. Br Med J. 1975 Jun 28;2(5973):714–715. doi: 10.1136/bmj.2.5973.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete M., Wittliff J. L., Schally A. V. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J Clin Lab Anal. 1989;3(3):137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- Fentiman I. S., Brame K., Chaudary M. A., Camplejohn R. S., Wang D. Y., Millis R. R. Perioperative bromocriptine adjuvant treatment for operable breast cancer. Lancet. 1988 Mar 19;1(8586):609–610. doi: 10.1016/s0140-6736(88)91413-4. [DOI] [PubMed] [Google Scholar]
- Fields K., Kulig E., Lloyd R. V. Detection of prolactin messenger RNA in mammary and other normal and neoplastic tissues by polymerase chain reaction. Lab Invest. 1993 Mar;68(3):354–360. [PubMed] [Google Scholar]
- Foekens J. A., Portengen H., van Putten W. L., Trapman A. M., Reubi J. C., Alexieva-Figusch J., Klijn J. G. Prognostic value of receptors for insulin-like growth factor 1, somatostatin, and epidermal growth factor in human breast cancer. Cancer Res. 1989 Dec 15;49(24 Pt 1):7002–7009. [PubMed] [Google Scholar]
- Freiss G., Rochefort H., Vignon F. Mechanisms of 4-hydroxytamoxifen anti-growth factor activity in breast cancer cells: alterations of growth factor receptor binding sites and tyrosine kinase activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):919–926. doi: 10.1016/s0006-291x(05)80873-3. [DOI] [PubMed] [Google Scholar]
- Ginsburg E., Vonderhaar B. K. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995 Jun 15;55(12):2591–2595. [PubMed] [Google Scholar]
- Grisoli F., Vincentelli F., Foa J., Lavail G., Salamon G. Effect of bromocriptine on brain metastasis in breast cancer. Lancet. 1981 Oct 3;2(8249):745–746. doi: 10.1016/s0140-6736(81)91065-5. [DOI] [PubMed] [Google Scholar]
- Holtkamp W., Nagel G. A., Wander H. E., Rauschecker H. F., von Heyden D. Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer. Int J Cancer. 1984 Sep 15;34(3):323–328. doi: 10.1002/ijc.2910340307. [DOI] [PubMed] [Google Scholar]
- Huynh H., Pollak M. Enhancement of tamoxifen-induced suppression of insulin-like growth factor I gene expression and serum level by a somatostatin analogue. Biochem Biophys Res Commun. 1994 Aug 30;203(1):253–259. doi: 10.1006/bbrc.1994.2175. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., Berns E. M., Foekens J. A. Prognostic factors and response to therapy in breast cancer. Cancer Surv. 1993;18:165–198. [PubMed] [Google Scholar]
- Klijn J. G., Berns P. M., Bontenbal M., Alexieva-Figusch J., Foekens J. A. Clinical breast cancer, new developments in selection and endocrine treatment of patients. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):211–221. doi: 10.1016/0960-0760(92)90210-a. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., Hoff A. M., Planting A. S., Verweij J., Kok T., Lamberts S. W., Portengen H., Foekens J. A. Treatment of patients with metastatic pancreatic and gastrointestinal tumours with the somatostatin analogue Sandostatin: a phase II study including endocrine effects. Br J Cancer. 1990 Oct;62(4):627–630. doi: 10.1038/bjc.1990.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijn J. G., Setyono-Han B., Bakker G. H., van der Burg M. E., Bontenbal M., Peters H. A., Sieuwerts A. M., Berns P. M., Foekens J. A. Growth factor-receptor pathway interfering treatment by somatostatin analogs and suramin: preclinical and clinical studies. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):1089–1095. doi: 10.1016/0960-0760(90)90471-v. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., de Jong F. H., Lamberts S. W., Blankenstein M. A. LHRH-agonist treatment in clinical and experimental human breast cancer. J Steroid Biochem. 1985 Nov;23(5B):867–873. doi: 10.1016/s0022-4731(85)80029-7. [DOI] [PubMed] [Google Scholar]
- Kopp A., Jonat W., Schmahl M., Knabbe C. Transforming growth factor beta 2 (TGF-beta 2) levels in plasma of patients with metastatic breast cancer treated with tamoxifen. Cancer Res. 1995 Oct 15;55(20):4512–4515. [PubMed] [Google Scholar]
- Lamberts S. W., Krenning E. P., Reubi J. C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991 Nov;12(4):450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Macleod R. M. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990 Apr;70(2):279–318. doi: 10.1152/physrev.1990.70.2.279. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., van der Lely A. J., de Herder W. W., Hofland L. J. Octreotide. N Engl J Med. 1996 Jan 25;334(4):246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- Lønning P. E., Hall K., Aakvaag A., Lien E. A. Influence of tamoxifen on plasma levels of insulin-like growth factor I and insulin-like growth factor binding protein I in breast cancer patients. Cancer Res. 1992 Sep 1;52(17):4719–4723. [PubMed] [Google Scholar]
- Malaab S. A., Pollak M. N., Goodyer C. G. Direct effects of tamoxifen on growth hormone secretion by pituitary cells in vitro. Eur J Cancer. 1992;28A(4-5):788–793. doi: 10.1016/0959-8049(92)90116-j. [DOI] [PubMed] [Google Scholar]
- Malarkey W. B., Kennedy M., Allred L. E., Milo G. Physiological concentrations of prolactin can promote the growth of human breast tumor cells in culture. J Clin Endocrinol Metab. 1983 Apr;56(4):673–677. doi: 10.1210/jcem-56-4-673. [DOI] [PubMed] [Google Scholar]
- Manni A., Boucher A. E., Demers L. M., Harvey H. A., Lipton A., Simmonds M. A., Bartholomew M. Endocrine effects of combined somatostatin analog and bromocriptine therapy in women with advanced breast cancer. Breast Cancer Res Treat. 1989 Dec;14(3):289–298. doi: 10.1007/BF01806300. [DOI] [PubMed] [Google Scholar]
- Manni A., Wright C., Davis G., Glenn J., Joehl R., Feil P. Promotion by prolactin of the growth of human breast neoplasms cultured in vitro in the soft agar clonogenic assay. Cancer Res. 1986 Apr;46(4 Pt 1):1669–1672. [PubMed] [Google Scholar]
- Mershon J., Sall W., Mitchner N., Ben-Jonathan N. Prolactin is a local growth factor in rat mammary tumors. Endocrinology. 1995 Aug;136(8):3619–3623. doi: 10.1210/endo.136.8.7628401. [DOI] [PubMed] [Google Scholar]
- Minton J. P. Proceedings: The response of breast cancer patients with bone pain to L-dopa. Cancer. 1974 Feb;33(2):358–363. doi: 10.1002/1097-0142(197402)33:2<358::aid-cncr2820330209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mol J. A., van Garderen E., Selman P. J., Wolfswinkel J., Rijinberk A., Rutteman G. R. Growth hormone mRNA in mammary gland tumors of dogs and cats. J Clin Invest. 1995 May;95(5):2028–2034. doi: 10.1172/JCI117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. J., Vrhovsek E., Sutherland R. L., Lazarus L. Growth hormone binding to cultured human breast cancer cells. J Clin Endocrinol Metab. 1984 Jan;58(1):149–156. doi: 10.1210/jcem-58-1-149. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Clemmons D. R., Arteaga C. L. Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):805–809. doi: 10.1016/0960-0760(90)90423-i. [DOI] [PubMed] [Google Scholar]
- Pekonen F., Partanen S., Mäkinen T., Rutanen E. M. Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res. 1988 Mar 1;48(5):1343–1347. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Beuscart R., Djiane J., Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer Res. 1988 Nov 15;48(22):6429–6433. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Hecquet B., Vennin P., Louchez M. M., Fournier C., Lefebvre J., Demaille A. Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur J Cancer. 1993;29A(4):492–497. doi: 10.1016/s0959-8049(05)80137-6. [DOI] [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Laurent J. C., Louchez M. M., Amrani S., Leroy-Martin B., Vilain M. O., Delobelle A., Demaille A. Presence and characterization of insulin-like growth factor 1 receptors in human benign breast disease. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1425–1431. doi: 10.1016/0277-5379(88)90332-x. [DOI] [PubMed] [Google Scholar]
- Pollak M. N., Huynh H. T., Lefebvre S. P. Tamoxifen reduces serum insulin-like growth factor I (IGF-I). Breast Cancer Res Treat. 1992;22(1):91–100. doi: 10.1007/BF01833337. [DOI] [PubMed] [Google Scholar]
- Pollak M. N., Polychronakos C., Guyda H. Somatostatin analogue SMS 201-995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res. 1989 Jul-Aug;9(4):889–891. [PubMed] [Google Scholar]
- Pollak M. N., Polychronakos C., Yousefi S., Richard M. Characterization of insulin-like growth factor I (IGF-I) receptors of human breast cancer cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):326–331. doi: 10.1016/0006-291x(88)90688-2. [DOI] [PubMed] [Google Scholar]
- Pollak M., Costantino J., Polychronakos C., Blauer S. A., Guyda H., Redmond C., Fisher B., Margolese R. Effect of tamoxifen on serum insulinlike growth factor I levels in stage I breast cancer patients. J Natl Cancer Inst. 1990 Nov 7;82(21):1693–1697. doi: 10.1093/jnci/82.21.1693. [DOI] [PubMed] [Google Scholar]
- Pratt S. E., Pollak M. N. Estrogen and antiestrogen modulation of MCF7 human breast cancer cell proliferation is associated with specific alterations in accumulation of insulin-like growth factor-binding proteins in conditioned media. Cancer Res. 1993 Nov 1;53(21):5193–5198. [PubMed] [Google Scholar]
- Prévost G., Hosford D., Thomas F. Receptors for somatostatin and somatostatin analogues in human breast tumors. Ann N Y Acad Sci. 1994 Sep 15;733:147–154. doi: 10.1111/j.1749-6632.1994.tb17264.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen C., Bergh T., Wide L., Brownell J. CV 205-502: a new long-acting drug for inhibition of prolactin hypersecretion. Clin Endocrinol (Oxf) 1987 Mar;26(3):321–326. doi: 10.1111/j.1365-2265.1987.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Christodoulides A., Koistinen R., Seppälä M., Teale J. D., Ghilchik M. W. The effect of endocrine therapy with medroxyprogesterone acetate, 4-hydroxyandrostenedione or tamoxifen on plasma concentrations of insulin-like growth factor (IGF)-I, IGF-II and IGFBP-1 in women with advanced breast cancer. Int J Cancer. 1992 Sep 9;52(2):208–212. doi: 10.1002/ijc.2910520209. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Waser B., Foekens J. A., Klijn J. G., Lamberts S. W., Laissue J. Somatostatin receptor incidence and distribution in breast cancer using receptor autoradiography: relationship to EGF receptors. Int J Cancer. 1990 Sep 15;46(3):416–420. doi: 10.1002/ijc.2910460315. [DOI] [PubMed] [Google Scholar]
- Rose D. P., Gottardis M., Noonan J. J. Rat mammary carcinoma regressions during suppression of serum growth hormone and prolactin. Anticancer Res. 1983 Sep-Oct;3(5):323–325. [PubMed] [Google Scholar]
- Santen R. J., Manni A., Harvey H., Redmond C. Endocrine treatment of breast cancer in women. Endocr Rev. 1990 May;11(2):221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Setyono-Han B., Henkelman M. S., Foekens J. A., Klijn G. M. Direct inhibitory effects of somatostatin (analogues) on the growth of human breast cancer cells. Cancer Res. 1987 Mar 15;47(6):1566–1570. [PubMed] [Google Scholar]
- Srkalovic G., Cai R. Z., Schally A. V. Evaluation of receptors for somatostatin in various tumors using different analogs. J Clin Endocrinol Metab. 1990 Mar;70(3):661–669. doi: 10.1210/jcem-70-3-661. [DOI] [PubMed] [Google Scholar]
- Szende B., Lapis K., Redding T. W., Srkalovic G., Schally A. V. Growth inhibition of MXT mammary carcinoma by enhancing programmed cell death (apoptosis) with analogs of LH-RH and somatostatin. Breast Cancer Res Treat. 1989 Dec;14(3):307–314. doi: 10.1007/BF01806302. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Gurd W., Lapointe M., Pollak M. Tamoxifen attenuates pulsatile growth hormone secretion: mediation in part by somatostatin. Endocrinology. 1992 Jun;130(6):3395–3401. doi: 10.1210/endo.130.6.1350760. [DOI] [PubMed] [Google Scholar]
- Vennin P., Peyrat J. P., Bonneterre J., Louchez M. M., Harris A. G., Demaille A. Effect of the long-acting somatostatin analogue SMS 201-995 (Sandostatin) in advanced breast cancer. Anticancer Res. 1989 Jan-Feb;9(1):153–155. [PubMed] [Google Scholar]
- Weber C., Merriam L., Koschitzky T., Karp F., Benson M., Forde K., LoGerfo P. Inhibition of growth of human breast carcinomas in vivo by somatostatin analog SMS 201-995: treatment of nude mouse xenografts. Surgery. 1989 Aug;106(2):416–422. [PubMed] [Google Scholar]
- Weckbecker G., Liu R., Tolcsvai L., Bruns C. Antiproliferative effects of the somatostatin analogue octreotide (SMS 201-995) on ZR-75-1 human breast cancer cells in vivo and in vitro. Cancer Res. 1992 Sep 15;52(18):4973–4978. [PubMed] [Google Scholar]
- Weckbecker G., Tolcsvai L., Stolz B., Pollak M., Bruns C. Somatostatin analogue octreotide enhances the antineoplastic effects of tamoxifen and ovariectomy on 7,12-dimethylbenz(alpha)anthracene-induced rat mammary carcinomas. Cancer Res. 1994 Dec 15;54(24):6334–6337. [PubMed] [Google Scholar]
- Winston R., Kao P. C., Kiang D. T. Regulation of insulin-like growth factors by antiestrogen. Breast Cancer Res Treat. 1994;31(1):107–115. doi: 10.1007/BF00689681. [DOI] [PubMed] [Google Scholar]
- van Eijck C. H., Krenning E. P., Bootsma A., Oei H. Y., van Pel R., Lindemans J., Jeekel J., Reubi J. C., Lamberts S. W. Somatostatin-receptor scintigraphy in primary breast cancer. Lancet. 1994 Mar 12;343(8898):640–643. doi: 10.1016/s0140-6736(94)92637-9. [DOI] [PubMed] [Google Scholar]
- van Roozendaal C. E., Klijn J. G., van Ooijen B., Claassen C., Eggermont A. M., Henzen-Logmans S. C., Foekens J. A. Transforming growth factor beta secretion from primary breast cancer fibroblasts. Mol Cell Endocrinol. 1995 Apr 28;111(1):1–6. doi: 10.1016/0303-7207(95)03539-j. [DOI] [PubMed] [Google Scholar]


