Abstract
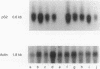
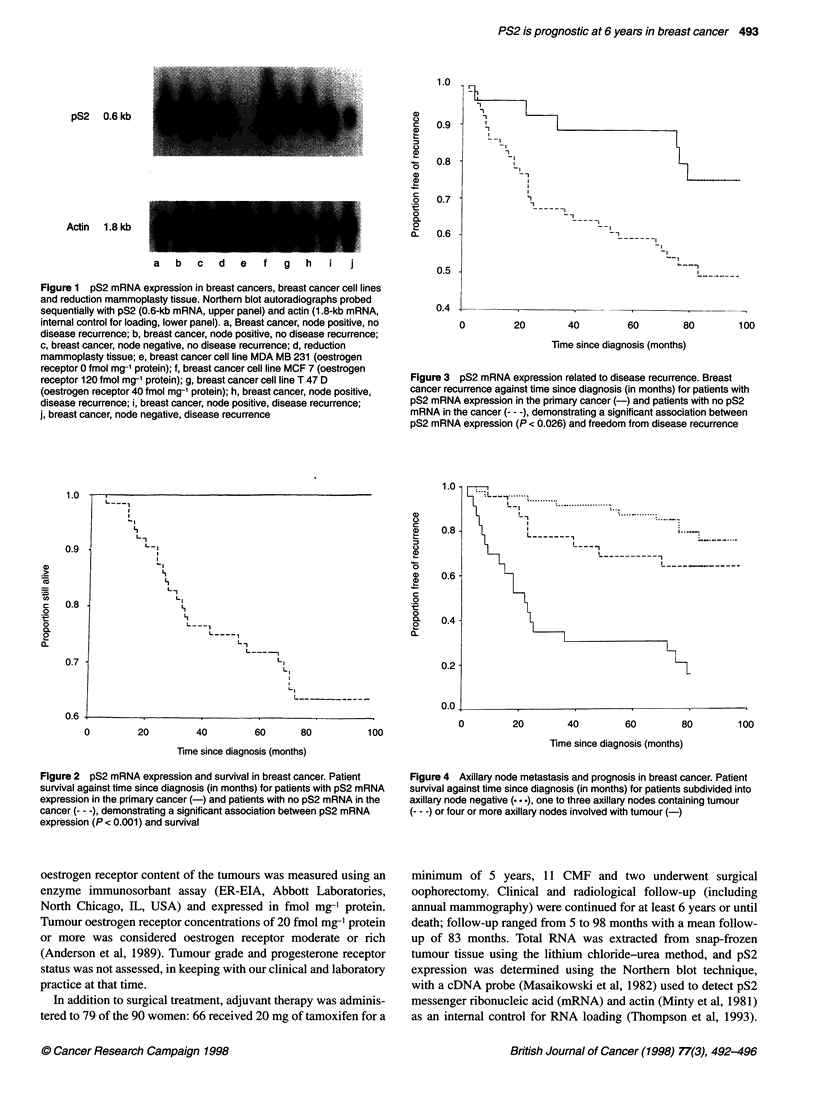
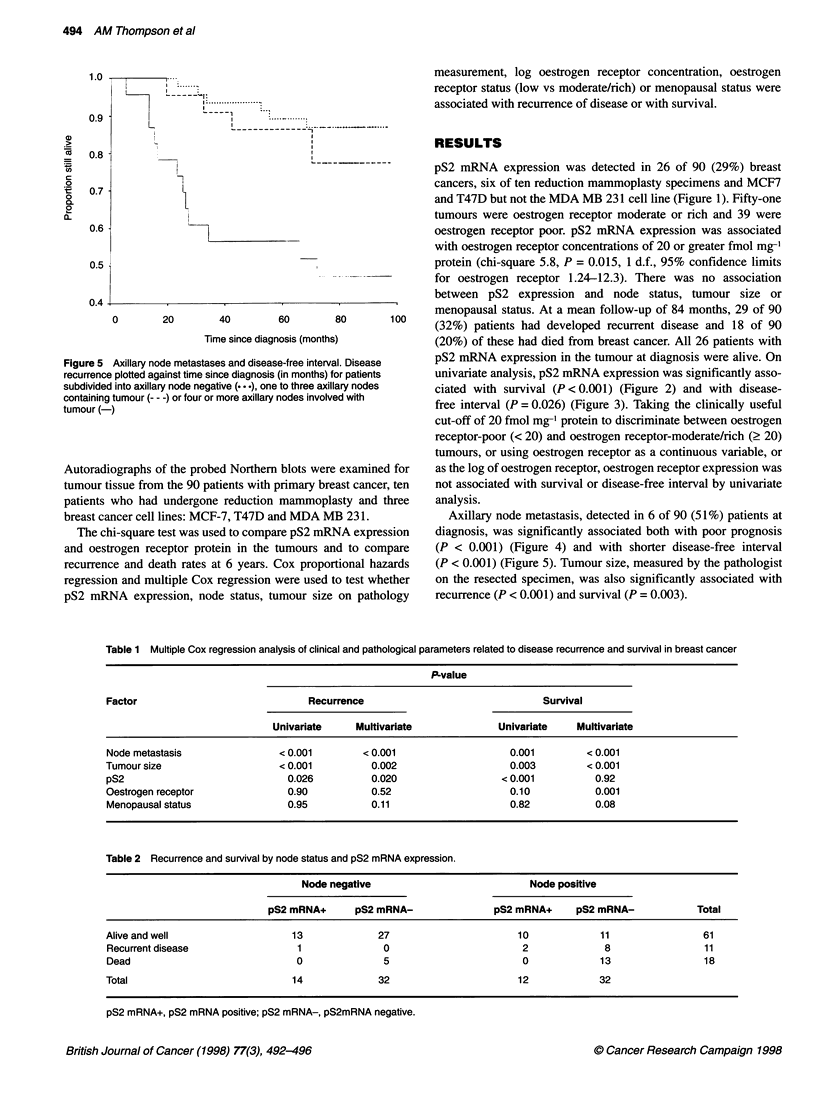
Expression of pS2, an oestrogen-regulated gene, has been associated with a good short-term prognosis and response to endocrine therapy. The aim of this study was to determine whether expression of mRNA for the pS2 gene in breast cancer could contribute useful information on disease behaviour and survival at medium-term follow-up. Northern blotting was used to detect pS2 messenger ribonucleic acid (mRNA) in the primary tumour tissue from each of 90 patients with breast cancer. Axillary node status was established by sampling or clearance, oestrogen receptor concentration by enzyme immunosorbant assay and follow-up was continued for at least 6 years or until death. At 83 months mean follow-up, 29 of 90 (32%) patients had recurrent disease and, of these, 18 (20%) had died from breast cancer. pS2 mRNA expression, present in 26 of 90 (29%) cancers, was associated with freedom from disease recurrence (P = 0.026) and was significantly associated with survival at a minimum of 6 years follow-up (P < 0.001). Pathological node status and tumour size were also significantly associated with disease recurrence (P < 0.001 and P = 0.002 respectively) and inversely with survival (P < 0.001 and P < 0.001 respectively). After multiple Cox regression analysis, pS2 expression was still a significant predictor of recurrence (but not survival) after adjusting for node status and tumour size; oestrogen receptor was an independent predictor of survival. The combination of node status and pS2 expression discriminated patients with particularly good prognosis (node negative, pS2 positive: no mortality at 6 years) or poor prognosis (node positive, pS2 negative; 41% mortality at 6 years). Evaluation of pS2 expression in breast cancer at diagnosis may provide additional useful prognostic information to conventional staging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. D., Forrest A. P., Levack P. A., Chetty U., Hawkins R. A. Response to endocrine manipulation and oestrogen receptor concentration in large operable primary breast cancer. Br J Cancer. 1989 Aug;60(2):223–226. doi: 10.1038/bjc.1989.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M., May F. E., Lennard T. W., Westley B. R. Determination of oestrogen responsiveness of breast cancer by competitive reverse transcription-polymerase chain reaction. Br J Cancer. 1995 Dec;72(6):1427–1434. doi: 10.1038/bjc.1995.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvenne C. G., Winkler-Gol R. A., Piccart M. J., Hustin J., Michaux D., Leclercq G., Nogaret J. M., Autier P. Expression of c-erbB2, TGF-beta 1 and pS2 genes in primary human breast cancers. Eur J Cancer. 1992;28(2-3):700–705. doi: 10.1016/s0959-8049(05)80130-3. [DOI] [PubMed] [Google Scholar]
- Detre S., King N., Salter J., MacLennan K., McKinna J. A., Dowsett M. Immunohistochemical and biochemical analysis of the oestrogen regulated protein pS2, and its relation with oestrogen receptor and progesterone receptor in breast cancer. J Clin Pathol. 1994 Mar;47(3):240–244. doi: 10.1136/jcp.47.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez Gibert O., Machuca I., Sebastián M. A., Rosel P., Navarro M. A. Expresión de la proteína pS2 en el cáncer de mama y su relación con los receptores de estrógenos y progesterona. Med Clin (Barc) 1996 Jun 15;107(3):90–92. [PubMed] [Google Scholar]
- Foekens J. A., Portengen H., Look M. P., van Putten W. L., Thirion B., Bontenbal M., Klijn J. G. Relationship of PS2 with response to tamoxifen therapy in patients with recurrent breast cancer. Br J Cancer. 1994 Dec;70(6):1217–1223. doi: 10.1038/bjc.1994.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens J. A., Rio M. C., Seguin P., van Putten W. L., Fauque J., Nap M., Klijn J. G., Chambon P. Prediction of relapse and survival in breast cancer patients by pS2 protein status. Cancer Res. 1990 Jul 1;50(13):3832–3837. [PubMed] [Google Scholar]
- Gion M., Mione R., Pappagallo G. L., Gatti C., Nascimben O., Bari M., Leon A. E., Vinante O., Bruscagnin G. PS2 in breast cancer--alternative or complementary tool to steroid receptor status? Evaluation of 446 cases. Br J Cancer. 1993 Aug;68(2):374–379. doi: 10.1038/bjc.1993.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Farndon J. R., Westley B. R., May F. E. Measurement of oestrogen receptor mRNA levels in human breast tumours. Br J Cancer. 1988 Nov;58(5):600–605. doi: 10.1038/bjc.1988.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Hennessy C., Lennard T. W., May F. E., Westley B. R. Expression of the oestrogen regulated pNR-2 mRNA in human breast cancer: relation to oestrogen receptor mRNA levels and response to tamoxifen therapy. Br J Cancer. 1990 Jan;61(1):32–38. doi: 10.1038/bjc.1990.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hähnel E., Joyce R., Sterrett G., Harvey J., Hähnel R. Detection of estradiol-induced messenger RNA (pS2) in uninvolved breast tissue from mastectomies for breast cancer. Breast Cancer Res Treat. 1992 Mar;20(3):167–176. doi: 10.1007/BF01834622. [DOI] [PubMed] [Google Scholar]
- Kardaś I., Seitz G., Limon J., Niezabitowski A., Ryś J., Theisinger B., Welter C., Blin N. Retrospective analysis of prognostic significance of the estrogen-inducible pS2 gene in male breast carcinoma. Cancer. 1993 Sep 1;72(5):1652–1656. doi: 10.1002/1097-0142(19930901)72:5<1652::aid-cncr2820720526>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kausitz J., Kuliffay P., Pecen L., Eben K., Puterová B. Correlation of cytosolic concentrations of ER, PS2, Cath-D, TPS, TK and cAMP in primary breast carcinomas. Neoplasma. 1994;41(6):331–336. [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Predine J., Spyratos F., Prud'homme J. F., Andrieu C., Hacene K., Brunet M., Pallud C., Milgrom E. Enzyme-linked immunosorbent assay of pS2 in breast cancers, benign tumors, and normal breast tissues. Correlation with prognosis and adjuvant hormone therapy. Cancer. 1992 Apr 15;69(8):2116–2123. doi: 10.1002/1097-0142(19920415)69:8<2116::aid-cncr2820690818>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Raam S., Robert N., Pappas C. A., Tamura H. Defective estrogen receptors in human mammary cancers: their significance in defining hormone dependence. J Natl Cancer Inst. 1988 Jul 20;80(10):756–761. doi: 10.1093/jnci/80.10.756. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. H., Koerner F. C., Edgerton S. M., Sawicka J. M., Rio M. C., Bellocq J. P., Chambon P., Thor A. D. pS2 expression and response to hormonal therapy in patients with advanced breast cancer. Cancer Res. 1991 Jan 15;51(2):624–628. [PubMed] [Google Scholar]
- Skilton R. A., Luqmani Y. A., McClelland R. A., Coombes R. C. Characterisation of a messenger RNA selectively expressed in human breast cancer. Br J Cancer. 1989 Aug;60(2):168–175. doi: 10.1038/bjc.1989.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran I., Quénel N., Mauriac L., Durand M., Bonichon F., Coindre J-M Variation of hormonal receptor, pS2, c-erbB-2 and GSTpi contents in breast carcinomas under tamoxifen: a study of 74 cases. Br J Cancer. 1996 Mar;73(6):735–743. doi: 10.1038/bjc.1996.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P., Stolzlechner J., Haider K., Heinzl H., Jakesz R., Pecherstorfer M., Rosen H., Sevelda P., Zeilliger R. pS2 protein status fails to be an independent prognostic factor in an average breast cancer population. Anticancer Res. 1994 Sep-Oct;14(5B):2125–2130. [PubMed] [Google Scholar]
- Stonelake P. S., Baker P. G., Gillespie W. M., Dunn J. A., Spooner D., Morrison J. M., Bundred N. J., Oates G. D., Lee M. J., Neoptolemos J. P. Steroid receptors, pS2 and cathepsin D in early clinically node-negative breast cancer. Eur J Cancer. 1994;30A(1):5–11. doi: 10.1016/s0959-8049(05)80008-5. [DOI] [PubMed] [Google Scholar]
- Thompson A. M., Hawkins R. A., Elton R. A., Steel C. M., Chetty U., Carter D. C. pS2 is an independent factor of good prognosis in primary breast cancer. Br J Cancer. 1993 Jul;68(1):93–96. doi: 10.1038/bjc.1993.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., May F. E. Estrogen-regulated messenger RNAs in human breast cancer cells. Cancer Treat Res. 1991;53:259–271. doi: 10.1007/978-1-4615-3940-7_12. [DOI] [PubMed] [Google Scholar]
- Wilson Y. G., Rhodes M., Ibrahim N. B., Padfield C. J., Cawthorn S. J. Immunocytochemical staining of pS2 protein in fine-needle aspirate from breast cancer is an accurate guide to response to tamoxifen in patients aged over 70 years. Br J Surg. 1994 Aug;81(8):1155–1158. doi: 10.1002/bjs.1800810824. [DOI] [PubMed] [Google Scholar]
- Wysocki S. J., Iacopetta B. J., Ingram D. M. Prognostic significance of pS2 mRNA in breast cancer. Eur J Cancer. 1994;30A(12):1882–1884. doi: 10.1016/0959-8049(94)00292-d. [DOI] [PubMed] [Google Scholar]
- Zaretsky J. Z., Weiss M., Tsarfaty I., Hareuveni M., Wreschner D. H., Keydar I. Expression of genes coding for pS2, c-erbB2, estrogen receptor and the H23 breast tumor-associated antigen. A comparative analysis in breast cancer. FEBS Lett. 1990 Jun 4;265(1-2):46–50. doi: 10.1016/0014-5793(90)80880-r. [DOI] [PubMed] [Google Scholar]