Abstract
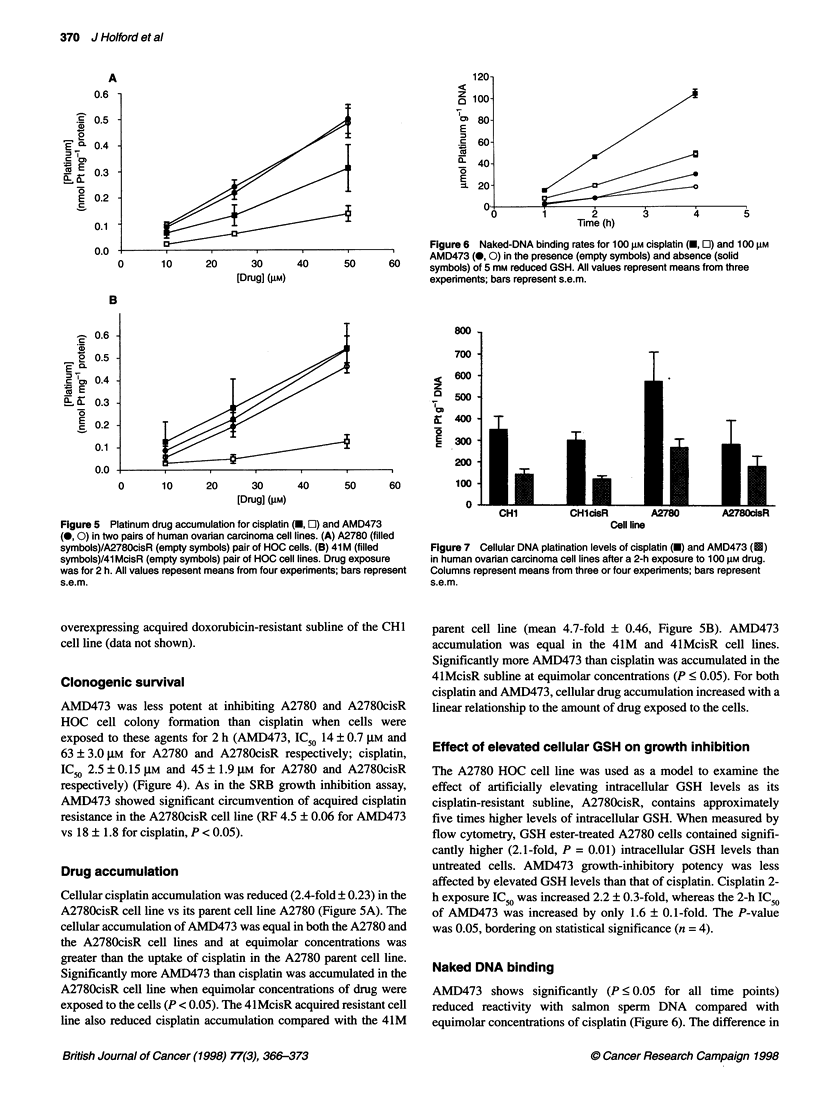
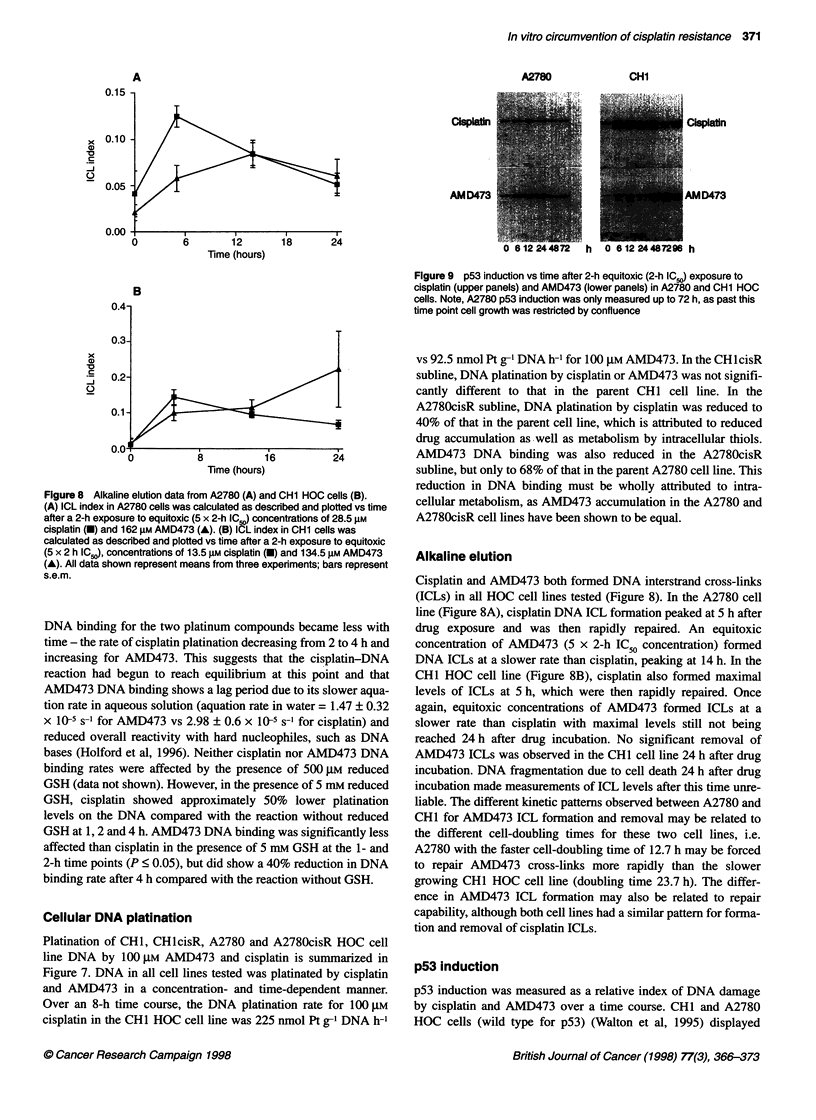
A novel sterically hindered platinum complex, AMD473 [cis-aminedichloro(2-methylpyridine) platinum (II)], has been selected for phase I clinical trials due to commence in 1997. AMD473 was rationally designed to react preferentially with nucleic acids over sulphur ligands such as glutathione. This report documents the in vitro circumvention of acquired cisplatin resistance mechanisms in human ovarian carcinoma (HOC) cell lines by AMD473. In a panel of 11 HOC cell lines, AMD473 showed intermediate growth inhibition potency (mean IC50 of 8.1 microM) in comparison to cisplatin (mean IC50 of 2.6 microM) and carboplatin (mean IC50 of 20.3 microM). AMD473 showed only a 30.7-fold increase in IC50 value from the most sensitive to the most resistant HOC cell line, whereas for cisplatin it was 117.9-fold and for carboplatin 119.7-fold. AMD473 also showed significantly (P < 0.05) reduced cross-resistance to cisplatin in a panel of three cell lines with known acquired platinum drug resistance mechanisms (mean RF for AMD473 was 1.9, for cisplatin 9.1). Cellular accumulation of AMD473 was not reduced in two HOC cell lines (A2780cisR and 41McisR), in which reduced cisplatin accumulation is a major mechanism of acquired cisplatin resistance. AMD473 naked-DNA binding was significantly less affected (P < 0.05) than that of cisplatin by the presence of 5 mM glutathione. Also, AMD473 almost completely circumvented acquired cisplatin resistance in a cell line (A2780cisR) with fivefold elevated intracellular glutathione levels compared with the parent A2780 cell line when measured by clonogenic assay (RF 4.5 for AMD473 vs RF 18 for cisplatin). AMD473 also showed a lower increase in IC50 than cisplatin in an A2780 cell line model with artificially elevated glutathione levels. AMD473 DNA binding was slower than that of cisplatin on both naked and cellular DNA. AMD473 also formed DNA interstrand cross-links (ICLs) at a slower rate than cisplatin (peak ICL formation was at 5 h for cisplatin vs > or = 14 h for AMD473) after equitoxic doses were exposed to HOC cells for 2 h. AMD473 ICLs in the CH1 HOC cell line were slowly formed and showed no visible signs of being repaired 24 h after removal of drug. This was paralleled by a slower, longer lasting induction of p53 protein by equitoxic doses of AMD473 in HOC cell lines with wild-type p53. This new class of sterically hindered platinum compound, selected for clinical trial in 1997, may therefore elicit improved clinical response in intrinsically and acquired cisplatin-resistant tumours in the clinic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens B. C., Hamilton T. C., Masuda H., Grotzinger K. R., Whang-Peng J., Louie K. G., Knutsen T., McKoy W. M., Young R. C., Ozols R. F. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987 Jan 15;47(2):414–418. [PubMed] [Google Scholar]
- Burchenal J. H., Kalaher K., Dew K., Lokys L. Rationale for development of platinum analogs. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1493–1498. [PubMed] [Google Scholar]
- Calsou P., Salles B. Role of DNA repair in the mechanisms of cell resistance to alkylating agents and cisplatin. Cancer Chemother Pharmacol. 1993;32(2):85–89. doi: 10.1007/BF00685607. [DOI] [PubMed] [Google Scholar]
- Delmastro D. A., Li J., Vaisman A., Solle M., Chaney S. G. DNA damage inducible-gene expression following platinum treatment in human ovarian carcinoma cell lines. Cancer Chemother Pharmacol. 1997;39(3):245–253. doi: 10.1007/s002800050568. [DOI] [PubMed] [Google Scholar]
- Gately D. P., Howell S. B. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993 Jun;67(6):1171–1176. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard P. M., Orr R. M., Valenti M. R., Barnard C. F., Murrer B. A., Kelland L. R., Harrap K. R. Novel trans platinum complexes: comparative in vitro and in vivo activity against platinum-sensitive and resistant murine tumours. Anticancer Res. 1996 Jan-Feb;16(1):33–38. [PubMed] [Google Scholar]
- Harrap K. R. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev. 1985 Sep;12 (Suppl A):21–33. doi: 10.1016/0305-7372(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994 Apr 1;15(4):349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- Hills C. A., Kelland L. R., Abel G., Siracky J., Wilson A. P., Harrap K. R. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989 Apr;59(4):527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Wright C. D., Ishizuka H. GS-X pump is functionally overexpressed in cis-diamminedichloroplatinum (II)-resistant human leukemia HL-60 cells and down-regulated by cell differentiation. J Biol Chem. 1994 Nov 18;269(46):29085–29093. [PubMed] [Google Scholar]
- Johnson S. W., Perez R. P., Godwin A. K., Yeung A. T., Handel L. M., Ozols R. F., Hamilton T. C. Role of platinum-DNA adduct formation and removal in cisplatin resistance in human ovarian cancer cell lines. Biochem Pharmacol. 1994 Feb 11;47(4):689–697. doi: 10.1016/0006-2952(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Fujiwara Y., Nishio K., Ohmori T., Sugimoto Y., Komiya K., Matsuda T., Saijo N. Metallothionein content correlates with the sensitivity of human small cell lung cancer cell lines to cisplatin. Cancer Res. 1991 Jun 15;51(12):3237–3242. [PubMed] [Google Scholar]
- Kelland L. R., Abel G., McKeage M. J., Jones M., Goddard P. M., Valenti M., Murrer B. A., Harrap K. R. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 1993 Jun 1;53(11):2581–2586. [PubMed] [Google Scholar]
- Kelland L. R., Barnard C. F., Evans I. G., Murrer B. A., Theobald B. R., Wyer S. B., Goddard P. M., Jones M., Valenti M., Bryant A. Synthesis and in vitro and in vivo antitumor activity of a series of trans platinum antitumor complexes. J Med Chem. 1995 Aug 4;38(16):3016–3024. doi: 10.1021/jm00016a004. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Barnard C. F., Mellish K. J., Jones M., Goddard P. M., Valenti M., Bryant A., Murrer B. A., Harrap K. R. A novel trans-platinum coordination complex possessing in vitro and in vivo antitumor activity. Cancer Res. 1994 Nov 1;54(21):5618–5622. [PubMed] [Google Scholar]
- Kelland L. R., Mistry P., Abel G., Loh S. Y., O'Neill C. F., Murrer B. A., Harrap K. R. Mechanism-related circumvention of acquired cis-diamminedichloroplatinum(II) resistance using two pairs of human ovarian carcinoma cell lines by ammine/amine platinum(IV) dicarboxylates. Cancer Res. 1992 Jul 15;52(14):3857–3864. [PubMed] [Google Scholar]
- Kelland L. R., Murrer B. A., Abel G., Giandomenico C. M., Mistry P., Harrap K. R. Ammine/amine platinum(IV) dicarboxylates: a novel class of platinum complex exhibiting selective cytotoxicity to intrinsically cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1992 Feb 15;52(4):822–828. [PubMed] [Google Scholar]
- Kelland L. R. New platinum antitumor complexes. Crit Rev Oncol Hematol. 1993 Dec;15(3):191–219. doi: 10.1016/1040-8428(93)90042-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Young R. C., Hamilton T. C. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst. 1989 Apr 5;81(7):535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Loh S. Y., Mistry P., Kelland L. R., Abel G., Harrap K. R. Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer. 1992 Dec;66(6):1109–1115. doi: 10.1038/bjc.1992.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamenta E. L., Poma E. E., Kaufmann W. K., Delmastro D. A., Grady H. L., Chaney S. G. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1994 Jul 1;54(13):3500–3505. [PubMed] [Google Scholar]
- McKeage M. J., Mistry P., Ward J., Boxall F. E., Loh S., O'Neill C., Ellis P., Kelland L. R., Morgan S. E., Murrer B. A phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol. 1995;36(6):451–458. doi: 10.1007/BF00685793. [DOI] [PubMed] [Google Scholar]
- Mellish K. J., Barnard C. F., Murrer B. A., Kelland L. R. DNA-binding properties of novel cis- and trans platinum-based anticancer agents in 2 human ovarian carcinoma cell lines. Int J Cancer. 1995 Sep 15;62(6):717–723. doi: 10.1002/ijc.2910620612. [DOI] [PubMed] [Google Scholar]
- Mellish K. J., Kelland L. R. Mechanisms of acquired resistance to the orally active platinum-based anticancer drug bis-acetato-ammine-dichloro-cyclohexylamine platinum (i.v.) (JM216) in two human ovarian carcinoma cell lines. Cancer Res. 1994 Dec 1;54(23):6194–6200. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Newkirk K., Sessions R. B., Andrews P. A., Trock B. J., Rasmussen A. A., Montgomery E. A., Bischoff E. K., Cullen K. J. Immunohistochemical staining for glutathione S-transferase predicts response to platinum-based chemotherapy in head and neck cancer. Clin Cancer Res. 1996 Nov;2(11):1859–1865. [PubMed] [Google Scholar]
- Pendyala L., Creaven P. J., Perez R., Zdanowicz J. R., Raghavan D. Intracellular glutathione and cytotoxicity of platinum complexes. Cancer Chemother Pharmacol. 1995;36(4):271–278. doi: 10.1007/BF00689042. [DOI] [PubMed] [Google Scholar]
- Sharp S. Y., Rogers P. M., Kelland L. R. Transport of cisplatin and bis-acetato-ammine-dichlorocyclohexylamine Platinum(IV) (JM216) in human ovarian carcinoma cell lines: identification of a plasma membrane protein associated with cisplatin resistance. Clin Cancer Res. 1995 Sep;1(9):981–989. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Bagrij T., Scheper R. J., Twentyman P. R. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br J Cancer. 1995 Jul;72(1):82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]