Abstract
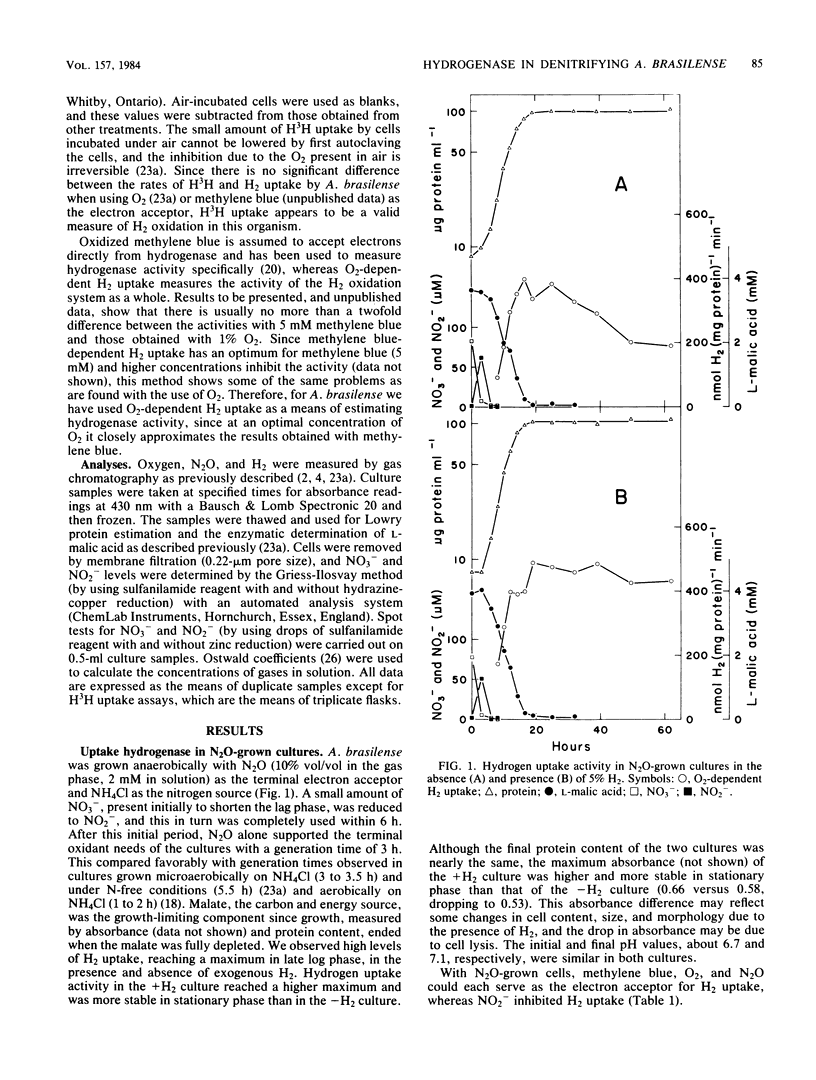
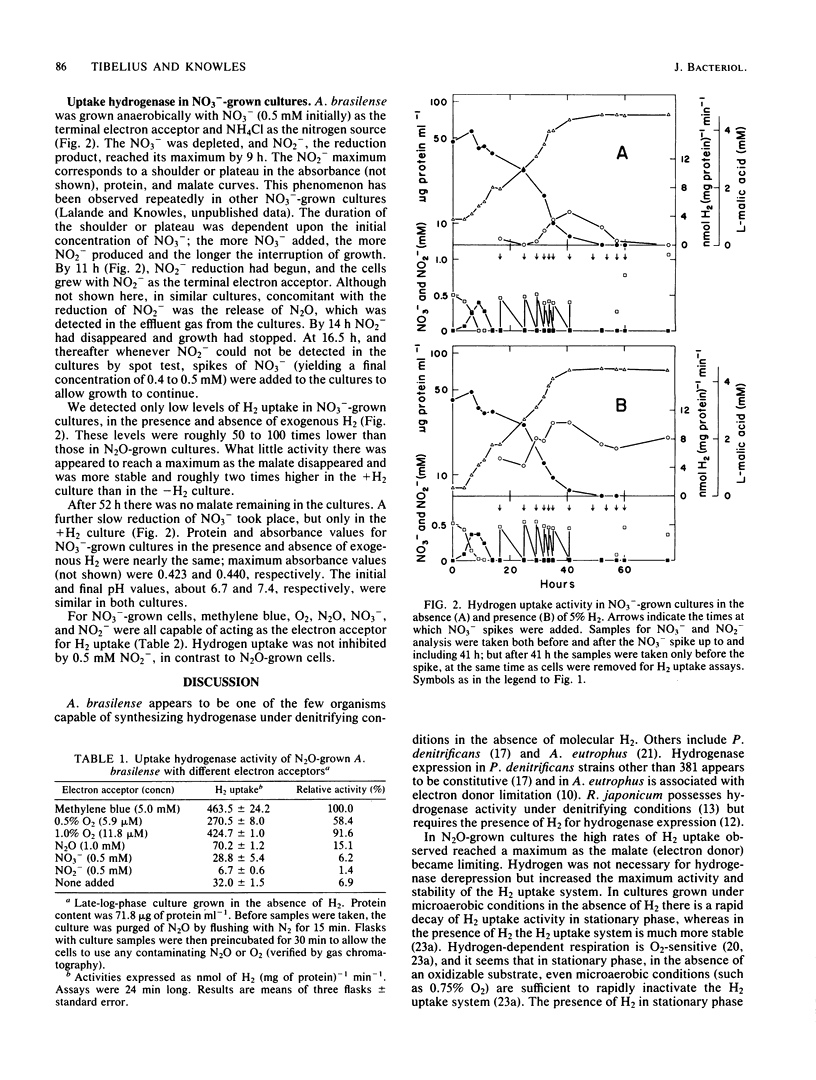
zospirillum brasilense Sp7 was grown anaerobically with N2O as the terminal electron acceptor and NH4Cl as the nitrogen source. Hydrogen uptake activity (O2-dependent H3H oxidation) was expressed in the presence and absence of 5% H2; it reached its maximum in late logarithmic phase as the malate became limiting. This activity was very stable in stationary phase, even in the absence of exogenous H2, compared with microaerobically grown cultures; this supports the hypothesis that the exclusion of O2 is critical for maintaining the integrity of the H2 uptake system in this organism. Oxygen, as well as methylene blue and N2O, supported H2 uptake, indicating the presence of electron transport components leading to O2 in anaerobically grown A. brasilense. Nitrite (0.5 mM) inhibited H2 uptake. In cultures grown with NO3- as the terminal electron acceptor and NH4Cl as the nitrogen source, in the presence and absence of exogenous H2, only low H2 uptake activity was observed. Methylene blue, O2, N2O, NO3-, and NO2- were all capable of acting as the electron acceptor for H2 oxidation. Nitrite (0.5 mM) did not inhibit H2 uptake in NO3--grown cells, as it did in N2O-grown cells. A. brasilense appears to be one of the few organisms capable of expressing the H2 uptake system under denitrifying conditions in the absence of molecular H2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTELLANI A. G., NIVEN C. F., Jr Factors affecting the bacteriostatic action of sodium nitrite. Appl Microbiol. 1955 May;3(3):154–159. doi: 10.1128/am.3.3.154-159.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. K., Knowles R. Measurement of denitrification in two freshwater sediments by an in situ acetylene inhibition method. Appl Environ Microbiol. 1979 Jun;37(6):1067–1072. doi: 10.1128/aem.37.6.1067-1072.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. K., Nelson L. M., Knowles R. Hydrogen metabolism of Azospirillum brasilense in nitrogen-free medium. Can J Microbiol. 1980 Sep;26(9):1126–1131. doi: 10.1139/m80-186. [DOI] [PubMed] [Google Scholar]
- Dobereiner J., Marriel I. E., Nery M. Ecological distribution of Spirillum lipoferum Beijerinck. Can J Microbiol. 1976 Oct;22(10):1464–1473. doi: 10.1139/m76-217. [DOI] [PubMed] [Google Scholar]
- Eskew D. L., Focht D. D., Ting I. P. Nitrogen fixation, denitrification, and pleomorphic growth in a highly pigmented Spirillum lipoferum. Appl Environ Microbiol. 1977 Nov;34(5):582–585. doi: 10.1128/aem.34.5.582-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G. Depression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982 Jan;149(1):203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUYVER A. J., VERHOEVEN W. Studies on-true dissimilatory nitrate reduction. IV. On adaptation in Micrococcus denitrificans. Antonie Van Leeuwenhoek. 1954;20(4):337–358. doi: 10.1007/BF02543738. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. M., Knowles R. Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol. 1978 Nov;24(11):1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- Neyra C. A., Döbereiner J. Denitrification by N2-fixing Sprillum lipoferum. Can J Microbiol. 1977 Mar;23(3):300–305. doi: 10.1139/m77-044. [DOI] [PubMed] [Google Scholar]
- Nokhal T. H., Schlegel H. G. The regulation of hydrogenase formation as a differentiating character of strains of Paracoccus denitrificans. Antonie Van Leeuwenhoek. 1980;46(2):143–155. doi: 10.1007/BF00444069. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Pfitzner J., Schlegel H. G. Denitrifikation bei Hydrogenomonas eutropha Stamm H 16. Arch Mikrobiol. 1973;90(3):199–211. [PubMed] [Google Scholar]
- Wodzinski R. S., Labeda D. P., Alexander M. Effects of low concentrations of bisulfite-sulfite and nitrite on microorganisms. Appl Environ Microbiol. 1978 Apr;35(4):718–723. doi: 10.1128/aem.35.4.718-723.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough J. M., Rake J. B., Eagon R. G. Bacterial inhibitory effects of nitrite: inhibition of active transport, but not of group translocation, and of intracellular enzymes. Appl Environ Microbiol. 1980 Apr;39(4):831–834. doi: 10.1128/aem.39.4.831-834.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Meijer E. M., Stouthamer A. H. Energy conservation during nitrate respiration in Paracoccus denitrificans. Arch Microbiol. 1977 Feb 4;112(1):17–23. doi: 10.1007/BF00446649. [DOI] [PubMed] [Google Scholar]



