Abstract
CO2 central chemoreceptors play an important role in cardiorespiratory control. They are highly sensitive to PCO2 in a broad range. These two sensing properties seem paradoxical as none of the known pH-sensing molecules can achieve both. Here we show that cultured neuronal networks are likely to solve the sensitivity versus spectrum problem with parallel and serial processes. Studies were performed on dissociated brainstem neurons cultured on microelectrode arrays. Recordings started after a 3 week initial period of culture. A group of neurons were dose-dependently stimulated by elevated CO2 with a linear response ranging from 20 to 70 Torr. The firing rate of some neurons increased by up to 30% in response to a 1 Torr PCO2 change, indicating that cultured brainstem neuronal networks retain high CO2 sensitivity in a broad range. Inhibition of Kir channels selectively suppressed neuronal responses to hypocapnia and mild hypercapnia. Blockade of TASK channels affected neuronal response to more severe hypercapnia. These were consistent with the pKa values measured for these K+ channels in a heterologous expression system. The CO2 chemosensitivity was reduced but not eliminated by blockade of presynaptic input from serotonin, substance P or glutamate neurons, indicating that both pre and postsynaptic neurons contribute to the CO2 chemosensitivity. These results therefore strongly suggest that the physiological PCO2 range appears to be covered by multiple sensing molecules, and that the high sensitivity may be achieved by cellular mechanisms via synaptic amplification in cultured brainstem neurons.
Spontaneous breathing requires continuous feedback controls by respiratory gases. Whereas O2 detection is carried out by peripheral chemoreceptors, CO2 sensing mainly depends on the central chemoreceptors (CCRs) known to be located in several brainstem areas (Feldman et al. 2003; Putnam et al. 2004; Richerson, 2004; Guyenet et al. 2005b). A mystery about the CCRs is how brainstem neurons manage to detect PCO2 as low as 1 mmHg, couple it to a 20–30% change in ventilation, and meanwhile cover a broad range of PCO2 (Nattie, 1999; Putnam et al. 2004). Sensitivity is an inherent property of CO2/pH sensing molecules that is determined by the steepness of the pH-response curve. A steep response, however, confines the sensing molecule to a narrow pH range. In contrast, a sensing molecule that covers a wide spectrum of PCO2 tends to have very low sensitivity. Indeed, none of the known CO2/pH-sensing molecules is capable of producing a change in membrane potentials or cellular activity by 20–30% in response to 1 mmHg PCO2 (Jiang et al. 2005). Exactly how the respiratory neuronal networks solve these seemingly paradoxical problems is unknown.
Several groups of brainstem neurons are CO2 chemosensitive, such as serotonergic neurons in the midline raphe nuclei, glutamatergic neurons in the retrotrapezoid nucleus, and catecholaminergic neurons in the locus coeruleus (Pineda & Aghajanian, 1997; Oyamada et al. 1998, 1999; Wang et al. 1998; Stunden et al. 2001; Filosa & Putnam, 2003; Severson et al. 2003; Mulkey et al. 2004; Ritucci et al. 2005). These neurons are not respiratory modulated, but they project to respiratory-modulated neurons, indicating that these CO2 chemosensitive cells are presynaptic (Richerson, 2004; Guyenet et al. 2005b). Brainstem respiratory-modulated neurons are also CO2 chemosensitive. In response to hypercapnia, inspiratory neurons are depolarized, and expiratory cells are hyperpolarized. These neuronal responses are retained after blockade of synaptic transmission, indicating that the postsynaptic cells are intrinsically CO2 chemosensitive (Onimaru et al. 1989; Kawai et al. 1996; Okada et al. 2002; Guyenet et al. 2005a; Kawai et al. 2006). The presence of CO2 chemosensitivity in both pre and postsynaptic neurons suggests a potential amplification mechanism by these neurons. Since these neurons are arranged in series in their network, we designated the neuronal process of CO2 signals as the serial process.
Several ion channels and receptors are modulated by physiological levels of PCO2/pH, and may act as sensors, including several Kir channels, TASK 1 channel, Ca2+ channels, TEA-sensitive K+ channels, gap junctions, P2x receptor, non-selective cationic channels, etc. (Dean et al. 1997; Thomas et al. 1999; Talley et al. 2000; Richerson et al. 2001; Putnam et al. 2004; Jiang et al. 2005). These potential CO2/pH sensing molecules coexist in several brainstem nuclei (Talley et al. 2000; Bradley et al. 2002; Washburn et al. 2002; Wu et al. 2004). Such a wide existence of these molecules suggests a process by which CO2 signals are detected by multiple sensing molecules working in parallel. We thereby named the latter neuronal process of CO2 signals the parallel process.
To find evidence for the serial and parallel processes, we performed studies in cultured brainstem neurons on microelectrode arrays (MEAs). The MEA technology allows recordings of neuronal activity in regular culture medium without evident perturbation of intra and extracellular environment such as temperature, pH, osmolarity, ionic composition, second messengers, etc. More importantly, the understanding of pre and postsynaptic processes of PCO2 signal requires simultaneous recordings from multiple neurons in their networks and de novo dissection of the synaptic mechanisms; the MEA is ideal for both in a reduced neuronal network (Su & Jiang, 2006).
Methods
Cell culture in microelectrode arrays
MEA dishes were purchased from ALA Scientific (Westbury, NY, USA), a distributor of MCS (Reutlingen, Germany), with each dish having 64 microelectrodes for which the tip diameter is 30 μm and the interelectrode space is 200 μm.
The preparation of primary neuronal culture was the same as we previously described (Su & Jiang, 2006). In brief, a timed-pregnant embryonic Sprague-Dawley rat (17–19 days) was anaesthetized with inhalation of saturated halothane (Halocarbon Laboratories, River Edge, NJ, USA), according to IACUC-approved protocols for the care and use of laboratory animals. Embryos were removed and chilled on ice. Under sterile conditions, all tissues from the lower brainstem containing the whole medulla and pons were cut into tissue blocks (0.5–1 mm3). From each fetus, 4–5 brainstem tissue pieces of ∼0.5 mm thickness were obtained, and each piece was split into two. The tissue pieces from all fetuses in one litter were mixed together and digested in a solution containing papain (Worthington-Biochem, Lakewood, NJ, USA) for 30 min in an incubator with 5% CO2 and 95% air, at 37°C. The digested tissue pieces were triturated by using a P-1000 Pipetman. Passing through a 40 μm Falcon filter (BD Biosciences, Bedford, MA, USA), the solution was centrifuged at 300 g for 5 min. After discontinuous density gradient centrifugation with albumin-inhibitor solution at 70 g for 6 min, the cell pellet was immediately resuspended in culture medium according to the protocol supplied by the manufacture (Worthington-Biochem). Dissociated cells (20 000–50 000) in a 20 μl droplet were plated onto the 1.5 mm2 electrode region of an MEA dish. With this protocol, each pregnant animal can be used for 8–10 MEA dishes. The dish was then filled with 1.2 ml neurobasal medium (NBM) supplemented with B 27 and GlutaMax I. The solution was changed to Dulbecco's modified Eagle's medium (DMEM) containing 10% horse serum (Gibco/Invitogen, Carlsbad, CA, USA) after 2 days. To reduce the variability among MEA dishes, great efforts were made to keep the cell mixture as homogeneous as possible, and recordings were performed under the same experimental conditions (Su & Jiang, 2006). However, there was certain variability among MEA dishes, and some dishes showed more responsive neurons than others; this is likely to be caused by the proportion of survival neurons from certain brainstem areas in each dish.
The MEA dish was covered with a Teflon MEA lid and tightly sealed with fluorinated ethylene-propylene membrane (Teflon FEP film, American Durafilm, Holliston, MA, USA). This film is permeable to CO2/O2, but not to microbes and water vapour (Potter & DeMarse, 2001). Cells were cultured at 36°C, with 5% CO2 and 95% air in a cell culture incubator (Model NU-4750; Nuaire, Plymouth, MN, USA).
MEA recording
Extracellular recordings were carried out at 36°C in DMEM by using a preamplifier (MEA1060-2; MCS) that held one MEA dish and was kept in an incubator during recording. Spikes were digitized at 40 kHz with a 64-channel A/D converter and the MEA Workstation software (Plexon, Dallas, TX, USA). Single-unit activity was then identified using the OfflineSorter software (Plexon) based on principal component analysis methods (Horn & Friedman, 2003). CO2 exposure was performed in a cell culture incubator in which the switch of CO2 was done by a CO2 controller and target level was reached within 1 min, as determined by the built-in CO2 sensor. At baseline, the chamber was ventilated with 5% CO2. CO2 exposures were achieved with different levels of PCO2 from 20 to 80 mmHg. A stretch of 10 min recordings was taken. Once the firing rate (FR) of baseline recording was stabilized in three consecutive records, exposures of step-elevated CO2 concentrations were performed subsequently, followed by 30–60 min washout with 5% CO2.
Neuronal response to hypercapnia was studied with at least three levels of PCO2 (50–80 mmHg). In response to hypercapnia, the firing activity of a group of units increased (see below), while others decreased or remained unchanged. To group these units, the peak response was measured first. Then, the sensitivity index C was determined: ΔFR/ΔPCO2, where ΔFR is the percentage change in the firing rate and ΔPCO2 is the change in PCO2 (mmHg). The value C = 0.0067 corresponds to a 0.67% change in FR per mmHg PCO2. Our measurements showed that extracellular pH (pHo) decreased from 7.42 to 7.13 with a PCO2 change from 38 to 76 mmHg or 13.2 mmHg PCO2/pHo 0.1 unit (Su & Jiang, 2006). Thus, our threshold C value equals an 8.8% change in FR per 0.1 unit pHo. Using this criterion, three groups of units were identified: CO2-stimulated units with C > 0.0067, CO2-inhibited units with C < −0.0067 and CO2-irresponsive units with 0.0067 > C > −0.0067. Furthermore, these response patterns had to be reproducible in at least two separate recordings within 24–72 h.
Neurotransmission blockade was performed with antagonist ketanserine tartrate for the 5 HT2A receptor, spantide acetate salt for the NK1 receptor, 6 cyano-7-nitroquinoxaline-2,3-dione (CNQX) for glutaminergic AMPA/kanate receptor, and pyridoxal phosphate-6-azophenyl-2,4-disulphonic acid tetrasodium salt (PPADS) for the purinergic P2x receptor. Blockade of Kir channels and TASK channels was also performed in the MEA system with Ba2+ (BaCl2) and anandamide in water-soluble emulsion (Tocrisolve 100), respectively (Maingret et al. 2001; Dwivedi et al. 2005; Orie et al. 2006). Control experiments were done before and after the chemical treatment with their solvent such as equally diluted Tocrisolve 100 for dissolving anandamide. Spantide was purchased from Sigma Chemicals (St Louis, MO, USA). All other chemicals were purchased from Tocris (Ellisville, MO, USA).
Immunocytochemistry
The cultured cells were processed for fluorescence immunocytochemical staining as previously described (Su & Jiang, 2006), and then examined under a confocal microscope (Zeiss LSM 510). The cells were incubated overnight with primary antibodies: rabbit polyclonal anti-Kir4.1 (1:1000), rabbit polyclonal anti-TASK 1 (1:500) (Alomone Laboratory, Israel), goat polyclonal anti-Kir5.1 (1:500; Santa Cruz Biotech, Santa Cruz, CA, USA), and mouse monoclonal anti-MAP2 (1:400; Sigma). Then double or triple labellings were performed by further incubations of cells with the secondary antibodies for 1.5 h: AlexaFluor488-conjugated donkey anti-rabbit IgG for showing Kir4.1 or TASK 1, AlexaFluor594-conjugated donkey anti-goat IgG for showing Kir5.1, AlexaFluor594-conjugated donkey anti-mouse IgG (1:1000; Molecular Probes, Eugene, OR, USA) or AMCA (7-amino-4-methylcoumarin-3-acetic acid) conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA, USA) for MAP2. Anti-GFAP (1:400; Sigma) is also used for identifying glial cells in some cultures. In control experiments, the primary antibodies were omitted for Kir5.1 and MAP2/GFAP, or preabsorbed with a threefold excess of the epitope for Kir4.1 (Alomone Laboratory). All of these control experiments showed negative staining.
Whole-cell voltage-clamp recording in Xenopus oocytes
Frog oocytes were obtained from Xenopus laevis, as previously described (Xu et al. 2000; Cui et al. 2001). Briefly, female frogs were deeply anaesthetized by bathing in 0.3% 3-aminobenzoic acid ethyl ester, according to IACUC-approved protocols. A few lobs of ovaries were removed and then treated with 0.5 mg ml−1 collagenase (Type I; Sigma) in OR2 solution containing (mm): NaCl 82, KCl 2, MgCl2 1 and Hepes 5 (pH 7.4) for 30 min at room temperature. After several washes with the OR2 solution, oocytes were injected with cDNAs (25–50 ng in 50 nL water) and then incubated at 18°C in ND-96 solution containing (mm): NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, Hepes 5, and sodium pyruvate 2.5, with 100 mg l−1 geneticin (pH 7.4).
Rat Kir4.1 (GenBank accession no. X83585) and rat Kir5.1 (GenBank accession no. X83581) cDNAs were generously provided by Dr John Adelman, Oregon Health and Science University. Mouse TASK1 cDNA (GenBank accession no. AAC53367) was a gift from Dr Michel Lazdunski, Institut de Pharmacologie Moleculaire et Cellulaire, France. A tandem Kir4.1–Kir5.1 was constructed, which expresses identical currents as those from coinjection of Kir4.1 and Kir5.1 (Xu et al. 2000; Yang et al. 2000). The cDNAs were subcloned into a eukaryotic expression vector, pcDNA3.1 (Invitrogen), and used for oocyte expression without cRNA synthesis.
Whole-cell currents were recorded by two-electrode voltage clamp 3 days after cDNA injection using an amplifier (Geneclamp 500; Axon Instruments, Union City, CA, USA) as previously detailed (Xu et al. 2000; Cui et al. 2001). Ba2+ and anandamide sensitivity was tested with extracellular exposure to various concentrations. The concentration–response relationships were expressed using the Hill equation, with IC50 determined (Yang & Jiang, 1999; Yang et al. 2000). TASK 1 channel sensitivity to extracellular pH was studied by using perfusates at various pH levels. Sensitivity of the Kir4.1–Kir5.1 channel to intracellular pH was studied in excised patches, as we have previously described (Yang & Jiang, 1999; Yang et al. 2000). The pH–current relationship was then described with the Hill equation.
Data are presented as means ± s.e.m. Differences between control and treatment groups were examined using ANOVA or Student's two-tailed paired t test, and considered to be statistically significant when P < 0.05.
Results
CO2 chemosensitivity of brainstem neurons cultured on MEAs
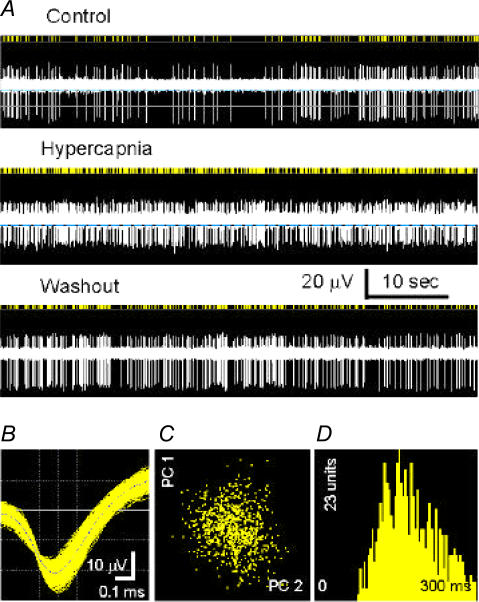
Neurons were obtained from the medulla oblongata and pons of fetal (day of pregnancy, 17–19) rats and cultured in MEA dishes. The general morphological properties of neurons, in which about 5% were serotonergic neurons, were the same as described in our first paper with the MEA technique (Su & Jiang, 2006). Glial cells were also present, and consisted of about 30% of the total cell numbers. Extracellular recording was carried out in DMEM at 36°C after 3 weeks of culture (Fig. 1A). Single-unit recordings were determined by the methods of principle component analysis (Horn et al. 2003) (Fig. 1B and C) and the absence of action potentials in the initial period of the interspike histogram (Fig. 1D). Most spikes showed a negative–positive waveform with a duration >1 ms, suggesting that they were recorded from the soma (Gustafsson & Jankowska, 1976; Jiang & Lipski, 1990; Su & Jiang, 2006). Spikes with small irregular amplitude and narrow duration (0.5–0.7 ms) were occasionally seen. They were considered to be recorded from axons, and they were rejected for further analysis.
Figure 1.
Recording from a single unit in a microelectrode array (MEA) dish A, the unit was reversibly stimulated with an exposure to 10% CO2. B, the digitized action potentials showed a duration >1 ms. Note that the longer positive wave is not shown. C, the principal component analysis (PCA) showed that all spikes detected were clustered in the X–Y axis system, where the X axis is PC2 (i.e. the waveform projection onto the first principal component) and Y axis is PC1 (i.e. the waveform projection onto the second principal component). D, the interspike interval histogram indicates single-unit recording because of the lack of action potentials in the initial 50 ms.
In response to hypercapnia, the activity of a group of units increased (Fig. 2A), while others decreased or remained unchanged (not shown). Based on the criteria described in Methods, we found 172 CO2-stimulated units, 195 CO2-inhibited units and 448 CO2-irresponsive units out of 815 units from total about 60 fetuses of 6 pregnant rats (see online Supplemental material, Supplemental Fig. 1). The existence of such three groups of cells is consistent with previous findings in brain slices and primary brainstem neuronal culture (Dean et al. 1989; Richerson, 1995; Wang et al. 1998). In the present study we focused on the CO2-stimulated units only. Some units lost their activity during experimental treatments and were not considered for data analysis.
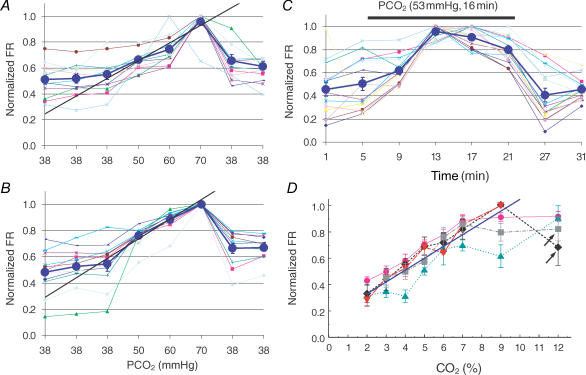
Figure 2.
Characterization of CO2-stimulated units A, in a MEA, CO2 reversibly augmented the firing rate (FR) of 11 units in a concentration-dependent manner (0.55 ± 0.04 to 0.96 ± 0.03; P < 0.001, n = 10, ANOVA). B, the response was reproducible as seen with repetitive exposure in a 24 h interval. Note that each symbol indicates a unit with the average of all units shown as large black circles (0.55 ± 0.05 to 1.00 ± 0.00, P < 0.001; n = 10, ANOVA). C, firing rate of 15 units in another MEA was augmented by 7% CO2, with the maximum response reached in 8 min (0.50 ± 0.06 to 0.80 ± 0.03, P < 0.001, n = 14, ANOVA). D, action potentials of the CO2-stimulated units from four MEA dishes, with one tested twice in 48 h (arrows), were normalized to the peak frequency of each unit during CO2 exposure, averaged in each dish, and displayed as means ± s.e.m. Note that the straight line indicates a linear change in FR with various levels of PCO2.
The CO2-stimulated units showed clear concentration-dependent responses to step changes of PCO2. When their firing activity was plotted against PCO2, an almost linear relationship was seen (Fig. 2A and B). The CO2 response was reversible and reproducible. Evident increase in firing activity of the CO2-stimulated units occurred in 3–5 min when the MEA dish was exposed to elevated CO2. The neuronal firing activity reached a maximum in 7–8 min and was maintained or showed a moderate decline during the rest of the CO2 exposure (Fig. 2C). The firing activity resumed to the baseline level in 30–60 min after switching back to 5% CO2. Thus, a 10 min CO2 exposure was used in the studies. Serial concentration–response curves were produced with 2, 3, 4, 5, 7, 9 and 12% CO2. The FR changed linearly around 5% CO2 (PCO2 38 mmHg) and reached the plateau at ∼9% CO2 (PCO2 68 mmHg) (Fig. 2D).
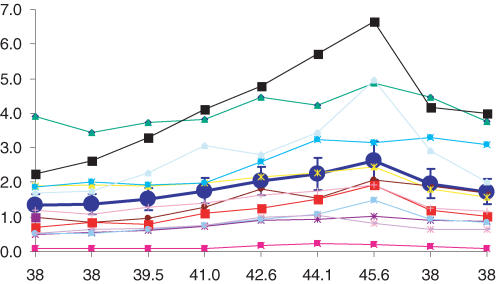
Some brainstem neurons in MEAs showed high CO2 sensitivity. To determine how high their CO2 sensitivity was, we studied their responses to modest changes of PCO2 in three MEA dishes. Twelve units were identified with C values of 0.03 or greater (0.064 ± 0.006, n = 12). In response to a 7.6 mmHg PCO2 change, the average FR of these units increased linearly from 1.36 ± 0.29 to 2.63 ± 0.56 Hz (P < 0.001, n = 11, ANOVA) (Fig. 3). This corresponds to a 16.7% change in FR per 1.0 mmHg PCO2, ranging from 4 to 30%, indicating that the CO2 chemosensitivity is well retained in the cultured brainstem neurons.
Figure 3.
Change in firing activity with modest CO2 changes Twelve highly CO2-chemosensitive units were selected from three MEA dishes. These units showed clear changes in their firing activity in response to step changes of CO2 by 1.5 mmHg. The large circles and thick line indicate a linear increase in firing rate from 1.3 to 2.6 Hz (P < 0.001, n = 11, ANOVA).
Parallel process of PCO2 signals with multiple pH-sensitive K+ channels
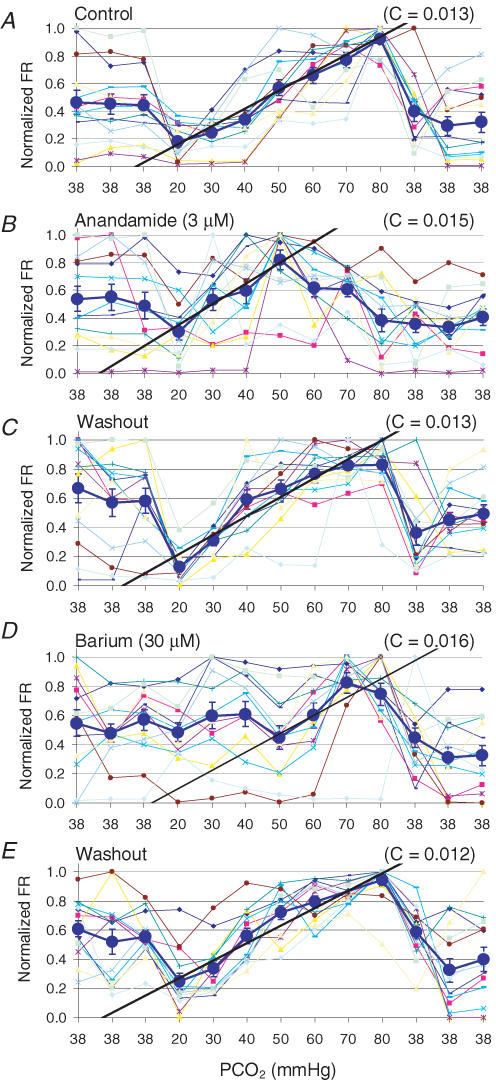
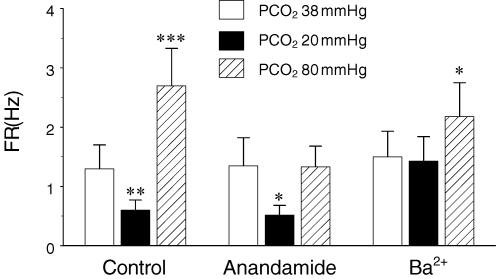
Several Kir and TASK channels are modulated by physiological levels of PCO2 and may act as CO2/pH sensors in brainstem neurons (Jiang et al. 2005). Selective blockade of the TASK 1 channels with 3 μm anandamide (Fig. 4B) (Maingret et al. 2001; Bai et al. 2006; Meuth et al. 2006) suppressed neuronal response to severe hypercapnia from 1.35 ± 0.47 to 1.33 ± 0.35 Hz (P > 0.05, n = 38, paired t test), while neuronal responses to moderate hypercapnia and hypocapnia were barely affected (from 1.35 ± 0.47 to 0.52 ± 0.16 Hz; P < 0.05, n = 38, paired t test) (Figs 4A and B, and 5). Blockade of the Kir channels with 30 μm Ba2+ (Fig. 4D) inhibited the responses of the same neurons to hypocapnia and mild hypercapnia (from 1.50 ± 0.43 to 1.43 ± 0.41 Hz; P > 0.05, n = 38, paired t test), whereas the FR of these neurons remained to be augmented by 70–80 mmHg PCO2, though to a less degree (from 1.50 ± 0.43 to 2.18 ± 0.57 Hz; P < 0.05, n = 38, paired t test) (Figs 4D and 5). The CO2 responses were mostly eliminated with high concentrations of Ba2+ (up to 500 μm) that block most Kir and TASK channels.
Figure 4.
Selective suppression of neuronal response to fractionalPCO2 A, in a MEA, a nearly linear relationship of the FR (in a 10 min period) with CO2 concentrations was seen as indicated by the straight line with a value ‘C’ showing the change in firing rate divided by the change in PCO2 (0.45 ± 0.08 to 0.93 ± 0.03; P < 0.001, n = 12, ANOVA). B, in the presence of 3 μm anandamide, neuronal sensitivity to PCO2 70–80 mmHg was abolished (0.49 ± 0.09 to 0.38 ± 0.08; P > 0.05, n = 12, ANOVA). D, in contrast, Ba2+ inhibited neuronal responses to PCO2 20–50 mmHg (0.53 ± 0.06 to 0.45 ± 0.08, P > 0.05, n = 12). C and E, evident recovery was seen with washout (0.58 ± 0.08 to 0.83 ± 0.05; P < 0.001, n = 12, ANOVA in C; 0.56 ± 0.04 to 0.94 ± 0.04; P < 0.001, n = 12, ANOVA in E).
Figure 5.
Summary of unit responses in the presence of anandamide and Ba2+ In the control, the average FR was significantly inhibited at PCO2 20 mmHg and augmented at PCO2 80 mmHg. With 3 μm anandamide, the neuronal response to hypercapnia, but not to hypocapnia, was lost. In the presence of 30 μm Ba2+, the hypocapnic response was suppressed, while neurons remained to be stimulated at PCO2 80 mmHg. Intra-group differences were examined by comparison to baseline FR (*P < 0.05; **P < 0.01; ***P < 0.001, n = 38, paired t test). Data were obtained from two MEAs.
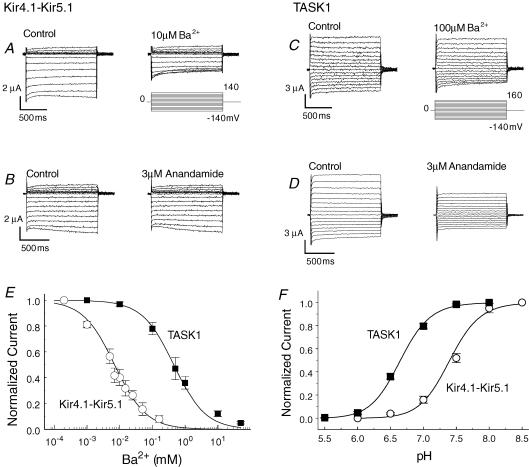
We chose TASK 1 and the Kir4.1–Kir5.1 channel, both of which are expressed in the brainstem neurons, to show the pH sensitivity of Kir and TASK channels (Talley et al. 2000; Washburn et al. 2002; Wu et al. 2004; also see Supplemental Fig. 2). These channels were expressed in Xenopus oocytes. Using whole-cell voltage clamp, the TASK 1 and Kir4.1–Kir5.1 channels were differentiated by their sensitivities to anandamide and Ba2+, respectively (Fig. 6A–E). The TASK1 channel showed extracellular pH sensitivity with pKa 6.65 (h = 1.9, n = 6; Fig. 6F). Since the Kir4.1–Kir5.1 channel is sensitive to intra but not extracellular pH (Xu et al. 2000), it was studied in inside-out patches. The Kir4.1–Kir5.1 channel was inhibited by intracellular acidification with pKa 7.40 (h = 2.1, n = 13; Fig. 6F). Therefore, these results are consistent with the neuronal responses to different levels of PCO2, further supporting that Kir and TASK channels may work in parallel and cover a wider PCO2 range than they can individually.
Figure 6.
Differential sensitivities of Kir4.1–Kir5.1 channel and TASK 1 channel to K+ channel blockers and pH A–D, whole-cell currents were studied in two-electrode voltage clamp in Xenopus oocytes. Ba2+ (10 μm) inhibited the Kir4.1–Kir5.1 (A) but not the TASK 1 channel (C). In contrast, the TASK 1 channel was more sensitive to anandamide (D) than the Kir4.1–Kir5.1 (B). E, the concentration–response relationship showed different Ba2+ sensitivity between the Kir4.1–Kir5.1 (IC50 = 7 μm, n = 4) and TASK 1 (IC50 = 450 μm, n = 4) channels. F, in Xenopus oocytes, the Kir4.1–Kir5.1 channel, both of which are expressed in the brainstem (Wu et al. 2004), was strongly inhibited at pH 7.0 (pKa = 7.40, h = 2.1, n = 13), while the TASK-1 channel was inhibited at more acidic pH (pKa = 6.65, h = 1.9, n = 6).
Our immunocytochemical assays in cultured brainstem neurons showed that Kir4.1 and Kir5.1 immunoreactivity was colocalized in the same MAP2-positive neurons, which was much denser than in the glial cells. The TASK 1 immunostaining was also localized in neurons, although glial cells also had positive stains (Supplemental Fig. 2).
Amplification of CO2 chemosensitivity by synaptic transmission
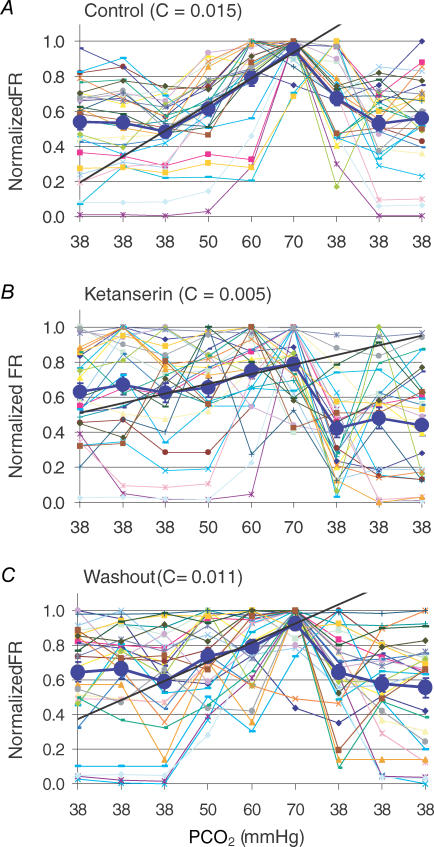
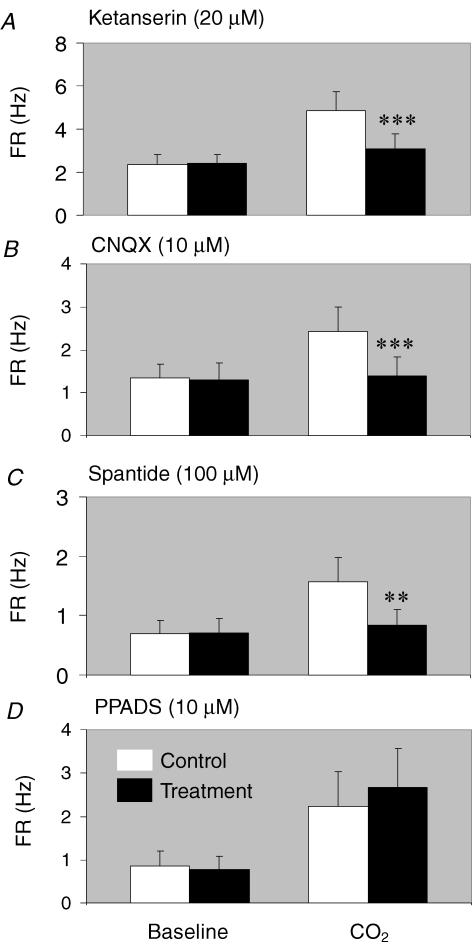
The high sensitivity of central chemoreceptors may be attributed to a potential amplification mechanism in the neuronal network. If this is the case, blockade of presynaptic input should reduce neuronal CO2 chemosensitivity. We thereby performed studies to test this hypothesis. Since several groups of serotonergic neurons in the brainstem are CO2 chemosensitive (Wang et al. 1998; Severson et al. 2003), we used 20 μm ketanserin to block 5 HT2A receptors known to play an important role in respiratory control (Richerson, 2004; Tryba et al. 2006). Ketanserin markedly diminished neuronal responses to hypercapnia from 4.88 ± 0.84 to 3.07 ± 0.69 Hz (P < 0.001, n = 28, paired t test) (Figs 7 and 8A). Amongst 29 CO2-stimulated units in three MEA dishes, only two cells lost their CO2 sensitivity completely. The CO2 chemosensitivity of such a small number of cells was interpreted to be derived solely from presynaptic input. The other 27 units remained to be stimulated by hypercapnia to various degrees. Of these 27 units, 20 showed significant reduction in their CO2 sensitivity, suggesting that the CO2 chemosensitivity of most neurons is determined by both pre and postsynaptic mechanisms. The CO2 chemosensitivity of the remaining seven units was either retained (n = 2) or slightly enhanced (n = 5) with ketanserin, suggesting that their CO2 chemosensitivity is independent of serotonergic input or slightly inhibited by serotonergic input.
Figure 7.
Changes in neuronal CO2 sensitivity before, during and after ketanserin (20 μM) exposure in two MEA dishes Neuronal FR was normalized to the peak frequency, and plotted against PCO2 levels. Ketanserin drastically attenuated neuronal response to hypercapnia (0.62 ± 0.06 to 0.79 ± 0.04, P < 0.05, n = 28, ANOVA).
Figure 8.
Effects of synaptic blockade on neuronal CO2 sensitivity A, when the average FR was compared with and without ketanserin, significant reductions in the FR were seen during CO2 (70 mmHg) exposure (P < 0.001, n = 28 from three MEAs, paired t test). B and C, Similar attenuations of the hypercapnic response were observed with CNQX (P < 0.001, n = 28 from four MEAs, paired t test) and spantide (P < 0.01, n = 21 from three MEAs, paired t test). D, PPADS, a P2x receptor blocker, did not have significant effect (P > 0.05, n = 36 from three MEAs, paired t test). Note that the units studied did not show significant differences in their baseline FR with or without the blockers (A–D).
Another group of candidate CO2 chemoreceptors are glutamatergic neurons, especially those in the retrotrapezoid nucleus (Mulkey et al. 2004). We found that neuronal CO2 sensitivity was significantly inhibited from 2.41 ± 0.57 to 1.40 ± 0.44 Hz (P < 0.001, n = 28, paired t test) in the presence of 10 μm CNQX, a non-NMDA receptor antagonist (23 reduced, 2 enhanced, and 4 lost response, out of 29 units in 4 MEAs) (Fig. 8B). A similar effect was found with blockade of NK1 receptors (from 1.57 ± 0.40 to 0.85 ± 0.25 Hz; P < 0.01, n = 21, paired t test) in the presence of 100 μm spantide (of 22 units in 3 MEAs, CO2 sensitivity was reduced in 17 units, enhanced in 3, and lost in 2; Fig. 8C). In contrast, blockade of purinergic P2x receptors that have been suggested to be involved in CO2 chemosensitivity (Thomas et al. 1999; Gourine et al. 2005) with 10 μm PPADS did not show any significant effect (from 2.24 ± 0.79 to 2.66 ± 0.92 Hz; P > 0.05, n = 36) (Fig. 8D), suggesting that the enhancement of CO2 chemosensitivity is rather specific to certain synaptic connections.
Discussion
In the present study, we have tested the hypothesis of serial and parallel processing of PCO2 signals using the MEA system. The MEA approach has several advantages for CO2 chemoreceptor studies. (1) We were able to record repetitively from multiple neurons with each individual unit identified by its firing pattern and CO2 response. (2) All recordings were done in cell culture media at 36°C, in which the neuronal extra and intracellular environments were not perturbed. (3) Neuronal CO2 chemosensitivity was studied with several levels of CO2 instead of a single dosage, allowing an estimation of the concentration–response relationship and the response pattern of individual neurons. (4) Action potentials were sorted with the method of principal component analysis before data analysis (Horn et al. 2003), so that the identification and isolation of single units was greatly improved over the traditional window discriminators. (5) Our results suggest that the neuronal inherent membrane properties and synaptic interaction necessary for CO2 chemosensitivity seem to be retained in such a reduced neuronal network. Although the neurons lost their surrounding tissues, they were more accessible for treatments with CO2 and other pharmacological agents. With the MEA system, we have shown evidence for neuronal processing of the CO2 signals in which the PCO2 spectrum seems to be covered by multiple sensing molecules and the sensitivity is enhanced with the CO2 chemosensitivity of both pre and postsynaptic neurons.
Our results have shown that ∼20% of cultured neurons in MEAs were stimulated by hypercapnia. The increase of the FR by elevated CO2 was reversible and reproducible. With step elevations in PCO2 levels, we have found that neurons in the MEA showed a linear response to the range of PCO2 from 20 to 80 mmHg, which is consistent with previous in vivo studies with respect to CO2 chemosensitivity (Nattie, 1999). Some neurons showed high CO2 sensitivity by changing their FR robustly in response to modest hypercapnia. Their average response is 16.7% per 1.0 mmHg PCO2, corresponding to ∼220% increase in FR per 0.1 pHo units, which falls in the upper range of CO2 sensitivity among all brainstem neurons (Putnam et al. 2004). These results suggest that cultured brainstem neurons still retain high CO2 chemosensitivity in a broad sensing spectrum as seen in other brainstem preparations (Putnam et al. 2004).
Previous studies have demonstrated the presence of CO2 chemosensitivity in both pre and postsynaptic neurons (Richerson, 1995; Kawai et al. 1996, 2006; Stunden et al. 2001; Takakura et al. 2006), which we inferred may allow an amplification of PCO2 signals detected by each cell. Consistent with this scenario, our results indicate that CO2 chemosensitivity is significantly reduced when 5-HT2A, NK1 or non-NMDA receptors are blocked, all of which are receptors for neurotransmitters that play an important role in the modulation of central respiratory activity and CO2 chemosensitivity (Feldman et al. 2003; Richerson, 2004; Guyenet et al. 2005b). Blockade of the P2x receptor, however, did not produce evident attenuation of the CO2 chemosensitivity, consistent with two recent studies showing lack of effect on the CO2 chemosensitivity by P2x receptor blockade (Lorier et al. 2004; Mulkey et al. 2006). It is possible that the brainstem neurons that are intrinsically CO2 chemosensitive may not receive direct purinergic synaptic input as suggested by Mulkey et al. (2006). It is worth emphasizing that the serial process should not be limited to two brainstem neurons. Instead, the signal amplification is likely to involve multiple interneurons, peripheral afferents and perhaps certain motoneurons as well, as some of them are pH sensitive as well (Harada et al. 1985; Talley et al. 2000; Takakura et al. 2006).
Several ion channels and receptors are modulated by physiological levels of PCO2/pH and may act as CO2/pH sensors in brainstem neurons (Dean et al. 1997; Thomas et al. 1999; Talley et al. 2000; Richerson, 2001; Putnam et al. 2004; Jiang et al. 2005). Our results suggest that some of these CO2/pH sensing molecules such as Kir channels and TASK channels play a part in detecting different levels of PCO2 in the same cells. While high levels of hypercapnia are likely to affect the TASK channels, the Kir channels appear to act in mild hypercapnia and hypocapnia. These observations are well supported by our whole-cell voltage-clamp studies of TASK 1 and Kir4.1–Kir5.1 channels in the Xenopus oocyte expression system, showing that the pH sensing ranges of these channels are consistent with the neuronal responses. Besides the Kir and TASK channels, our experiments do not exclude the possibility that other sensing molecules may play a similar role in the CO2 chemosensitivity. The wide distribution of multiple CO2/pH sensing molecules may thus allow brainstem neurons to cover the PCO2 spectrum using a similar parallel process.
In conclusion, although a good number of brainstem neurons are inherently CO2 chemosensitive, their CO2 sensitivity does not seem adequate to produce a 20–30% change in systemic respiratory response to 1 mmHg PCO2 Our results suggest that the physiological range of PCO2 seems to be covered by multiple CO2/pH sensing molecules that respond to different levels of PCO2, and the CO2 chemosensitivity is strongly augmented with synaptic transmission involving 5-HT2A, NK1 and non-NMDA receptors. Therefore, the spectrum problem appears to be solved by a parallel process of the PCO2 signals by multiple sensing molecules, and the sensitivity problem is eased with cellular mechanisms involving synaptic amplifications by CO2-chemosensitive neurons arranged in serial in cultured brainstem neurons.
Acknowledgments
This work was supported by an NIH grant (HL067890).
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.115758
http://jp.physoc.org/cgi/content/full/jphysiol.2006.115758/DC1 and contains supplemental material consisting of two figures:
Summary of sensitive index of CO2-stimulated, CO2-unresponsive and CO2-inhibited neuron
Immunocytochemical staining ofKir4.1, Kir5.1 and TASK1 channels in cultured brainstem cells
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Bai XL, Lacey HA, Greenwood SL, Baker PN, Turner MA, Sibley CP, Fyfe GK. TASK channel expression in human placenta and cytotrophoblast cells. J Soc Gynecol Invest. 2006;13:30–39. doi: 10.1016/j.jsgi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang WG, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Cui N, Giwa LR, Xu H, Rojas A, Abdulkadir L, Jiang C. Modulation of the heteromeric Kir4.1-Kir5.1 channels by PCO2 at physiological levels. Am J Physiol Cell Physiol. 2001;189:C229–C236. doi: 10.1002/jcp.10021. [DOI] [PubMed] [Google Scholar]
- Dean JB, Huang RQ, Erlichman JS, Southard TL, Hellard DT. Cell-cell coupling occurs in dorsal medullary neurons after minimizing anatomical-coupling artifacts. Neuroscience. 1997;80:21–40. doi: 10.1016/s0306-4522(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Dwivedi R, Saha S, Chowienczyk PJ, Ritter JM. Block of inward rectifying K+ channels (K-IR) inhibits bradykinin-induced vasodilatation in human forearm resistance vasculature. Arterioscler Thromb Vasc Biol. 2005;25:E7–E9. doi: 10.1161/01.ATV.0000152610.40086.31. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Harada Y, Wang YZ, Kuno M. Central chemosensitivity to H+ and CO2 in the rat respiratory center in vitro. Brain Res. 1985;333:336–339. doi: 10.1016/0006-8993(85)91588-4. [DOI] [PubMed] [Google Scholar]
- Horn CC, Friedman MI. Detection of single unit activity from the rat vagus using cluster analysis of principal components. J Neurosci Methods. 2003;122:141–147. doi: 10.1016/s0165-0270(02)00304-7. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang RP, Wang XR. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol. 2004;142:27–42. doi: 10.1016/j.resp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69:1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res. 1989;76:530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- Orie NN, Fry CH, Clapp LH. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc Res. 2006;69:107–115. doi: 10.1016/j.cardiores.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Andrzejewski M, Muckenhoff K, Scheid P, Ballantyne D. Locus coeruleus neurones in vitro: pH-sensitive oscillations of membrane potential in an electrically coupled network. Respir Physiol. 1999;118:131–147. doi: 10.1016/s0034-5687(99)00088-2. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem–spinal cord of the neonatal rat. J Physiol. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110:17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang WG, Tiwari J, Bradley SR. Chemo sensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang WG, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Su J, Jiang C. Multicellular recordings of cultured brainstem neurons in microelectrode arrays. Cell Tissue Res. 2006;326:25–33. doi: 10.1007/s00441-006-0228-y. [DOI] [PubMed] [Google Scholar]
- Takakura ACT, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei QB, Sirois JE, Bayliss DA. TASK 1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Jiang C. Opposite effects of pH on open-state probability and single channel conductance of kir4.1 channels. J Physiol. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, Shen W, Jiang C. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1–Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of sensitive index of CO2-stimulated, CO2-unresponsive and CO2-inhibited neuron
Immunocytochemical staining ofKir4.1, Kir5.1 and TASK1 channels in cultured brainstem cells