Abstract
Isoxyl (ISO), a thiourea derivative that was successfully used for the clinical treatment of tuberculosis during the 1960s, is an inhibitor of the synthesis of oleic and mycolic acids in Mycobacterium tuberculosis. Its effect on oleic acid synthesis has been shown to be attributable to its inhibitory activity on the stearoyl-coenzyme A desaturase DesA3, but its enzymatic target(s) in the mycolic acid pathway remains to be identified. With the goal of elucidating the mode of action of ISO, we have isolated a number of spontaneous ISO-resistant mutants of M. tuberculosis and undertaken their genotypic characterization. We report here the characterization of a subset of these strains carrying mutations in the monooxygenase gene ethA. Through complementation studies, we demonstrate for the first time that the EthA-mediated oxidation of ISO is absolutely required for this prodrug to inhibit its lethal enzymatic target(s) in M. tuberculosis. An analysis of the metabolites resulting from the in vitro transformation of ISO by purified EthA revealed the occurrence of a formimidamide allowing the formulation of an activation pathway in which the oxidation of ISO catalyzed by EthA is followed by chemical transformations involving extrusion or elimination and, finally, hydrolysis.
Mycobacterium tuberculosis, the etiologic agent of tuberculosis (TB), claims about 1.7 million lives annually, and the global number of TB cases is still rising at a rate of 0.6% per year (28). Approximately 2% of new TB cases in the world are attributed to multidrug-resistant strains, defined as M. tuberculosis isolates resistant to at least isoniazid and rifampin, the two most powerful anti-TB drugs. However, multidrug resistance rates in some countries, particularly in the former Soviet Union, Asia, the Dominican Republic, and Argentina, are much higher and may reach more than 22% of all isolates (16). Moreover, extensively drug-resistant strains that are resistant to three or more of the six classes of second-line drugs in addition to rifampin and isoniazid have been reported in all regions of the world (5), raising the possibility of future epidemics of virtually untreatable forms of TB. The continuing rise in disease incidence, the problem of drug resistance, and the need to reduce treatment duration has prompted research on new drug developments (11). In this light, the reexamination of isoxyl (ISO) (4,4′-diisoamyloxydiphenylthiourea; 4,4′-diisoamyloxythiocarbanilide; thiocarlide), a thiourea derivative that was successfully used for the clinical treatment of TB during the 1960s, may prove useful in identifying new therapeutic targets and in developing new therapeutic agents with greater potency and more desirable features than earlier thioureas (1, 14, 21, 23-24).
An early article reported that ISO inhibits the synthesis of mycolic acids and free fatty acids in Mycobacterium bovis BCG (27). We later demonstrated that ISO displays potent activity against other slow- and fast-growing species of Mycobacterium, including multidrug-resistant clinical isolates of M. tuberculosis, and showed that the drug affects the synthesis of all types of mycolic acids in addition to that of shorter-chain fatty acids in M. bovis BCG, M. tuberculosis H37Rv, and Mycobacterium aurum A+ (17). Our recent evidence indicates that the main effect of ISO in fatty acid metabolism is in the inhibition of the synthesis of oleic acid and that this effect is directly attributable to the inhibitory effect of the drug on the membrane-associated stearoyl-coenzyme A (CoA) (Δ9) desaturase DesA3 (Rv3229c) (18). Interestingly, sterculic acid, a known inhibitor of membrane-associated Δ9 desaturases, emulated the effect of ISO on oleic acid synthesis but did not affect mycolic acid synthesis, demonstrating that there is no relationship between the two effects of the drug (18). Therefore, ISO has at least one other enzymatic target in the mycolic acid biosynthetic pathway. This assumption is also supported by the fact that among the ISO derivatives that have been synthesized, some of the most potent ones still affected mycolic acid synthesis while having lost the ability to inhibit that of oleic acid (4).
Several lines of evidence suggest that ISO is a prodrug requiring prior metabolic activation for antimycobacterial activity. ISO has a long history of cross-resistance with ethionamide (ETH) and thiacetazone (TAC), two second-line anti-TB drugs that share with ISO a thiocarbonyl moiety which requires S oxidation for expression of their toxicity. Genetic analysis of a subset of cross-resistant M. tuberculosis isolates identified the flavin-containing monooxygenase EthA (Rv3854c) as the likely common activator of all three drugs (6). This assumption was more recently substantiated by the demonstration that EthA catalyzes the direct enzymatic transformation of ETH, TAC, and ISO in vitro and that of ETH in vivo (6, 8-9, 12, 19, 25). Furthermore, ethA overexpression was shown to increase the sensitivity of Mycobacterium smegmatis or M. bovis BCG to ETH, TAC, and ISO, while overexpression of ethR (Rv3855), a repressor of ethA, conferred ETH resistance on M. tuberculosis and M. smegmatis and both ETH and TAC resistance on M. bovis BCG (2, 6, 8). Interestingly, however, ethR overexpression had no effect on the susceptibilities of M. smegmatis, M. bovis BCG, and M. tuberculosis to ISO, and the disruption of the ethR gene in M. bovis BCG increased the sensitivity of the mutant strain to the drug only 2-fold, compared to 10-fold in the case of ETH and TAC (2, 6, 8). Consequently, in spite of clinical and experimental observations suggesting that ISO is a prodrug that requires EthA for activation, the likely existence of multiple cellular targets of this drug, all of which may not be targeted by the same metabolite of ISO, and the modest effect of ethR expression levels on the susceptibility of mycobacteria to this compound raised questions about the absolute requirement of ISO activation for mycobacteriostatic activity and the role of EthA in this process. The identity of the metabolite(s) resulting from the activation of ISO by EthA also remained to be determined.
With the goal of elucidating the mode of action of ISO, we have isolated a number of M. tuberculosis strains displaying resistance to this drug and have undertaken their genotypic and phenotypic characterization. We report here the characterization of a subset of these strains carrying mutations in the ethA gene and describe the effects of purified EthA on the metabolic transformation and activity of ISO in cell-free assays.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. tuberculosis H37Rv (ATCC 25618), the mutT1 transposon mutant of M. tuberculosis Mt103 (MT1K) (7), and M. bovis BCG 1173P2 (Pasteur strain) were grown in Middlebrook 7H9 medium (Difco) supplemented with ADC (0.2% dextrose, 0.5% bovine serum albumin fraction V, 0.085% NaCl, 0.0003% beef catalase) and 0.05% Tween 80 or on solid Middlebrook 7H11 medium (Difco) supplemented with OADC (0.005% oleic acid, 0.2% dextrose, 0.5% bovine serum albumin fraction V, 0.085% NaCl, 0.0003% beef catalase). Escherichia coli XL1-blue, the strain used for cloning experiments, was propagated in Luria-Bertani broth (pH 7.5) (Bactotryptone [10 g/liter], Bacto yeast extract [5 g/liter], NaCl [5 g/liter]) (Becton Dickinson, Sparks, MD). M. smegmatis mc2155 was grown in Middlebrook 7H9 medium (Difco) supplemented with ADC and 0.05% Tween 80, Luria-Bertani broth supplemented with 0.05% Tween 80, or MM63 minimal medium supplemented with 0.025% tyloxapol. Where indicated, kanamycin and hygromycin B were added to final concentrations of 20 μg/ml and 50 μg/ml, respectively.
Synthesis of ISO.
The starting material in our synthesis of ISO was the commercially available p-nitrophenol. Alkylation of p-nitrophenol with 1-bromo-3-methyl-butane (isoamyl bromide) in the presence of 18-crown-6 ether and anhydrous potassium carbonate in acetone (20) and subsequent reduction with tin chloride in ethanol (3) gave p-isoamyloxy aniline. The yields in this two-step synthesis were high (87% in each case). Treatment of p-isoamyloxy aniline with carbon disulfide in pyridine in the presence of diphenyl phosphite and subsequent column chromatography of the crude product yielded the pure ISO. Nuclear magnetic resonance and mass spectrometry data support the structure of the product.
Drug susceptibility testing.
MICs of ISO against M. tuberculosis wild-type and ISO-resistant strains were determined using the colorimetric resazurin microtiter assay (15) in 7H9-ADC broth at 37°C in the presence of 2% dimethyl sulfoxide (DMSO). The susceptibilities of the same strains to ethionamide were determined by the agar dilution method on 7H11-OADC medium containing 0, 1, 2.5, or 10 μg/ml of ethionamide (17). The MICs of ISO against M. tuberculosis H37Rv overexpressing ethA or ethR were determined by using the resazurin microtiter assay with 7H9-ADC broth at 32°C (ethA) or 37°C (ethR).
PCR amplification and DNA sequencing.
The entire promoter and coding sequences of desA3, ethA, and ethR were amplified from M. tuberculosis genomic DNA of wild-type and mutant strains using standard PCR strategies with Pfu DNA polymerase (Stratagene) and sequenced using a capillary Applied Biosystems ABI 3100 genetic analyzer. The pairs of primers used for PCR were (5′-GTGGTCGACCTGGTGGAAGGC-3′) and (5′-GTCAAGTCTGGCTGAGCACTG-3′) for desA3, (5′-TGTCGGAGTCGGAGCGAATTCC-3′) and (5′-GCATCGACCGAGCACCCCCGA-3′) for ethR, and (5′-AGCGGACGGTCCTCGAGAAGG-3′) and (5′-ACGGCATCATCGTCGTCTGAC-3′) for ethA.
Overexpression of ethA and ethR in M. tuberculosis.
The ethA and ethR genes were PCR amplified with Pfu DNA polymerase (Stratagene) from M. tuberculosis H37Rv genomic DNA using primers ethA.1 (5′-CGCCCGGCATATGACCGAGCACCTCGACGTTG-3′) and ethA.2 (5′-CGCAAGCTTAACCCCCACCGGGGCAGGCC-3′) and ethR.1 (5′-GGGAAACATATGACCACCTCCGCGGCCAGTCAG-3′) and ethR.2 (5′-CCCAAGCTTGCGGTTCTCGCCGTAAATGCT-3′) and placed under control of the hsp60 transcription and translation signals in the mycobacterial expression plasmid pVV16 (13), yielding pVVethA and pVVethR. Recombinant proteins produced with this system carry a six-histidine tag at their carboxyl terminus, allowing their analysis by immunoblotting with a mouse monoclonal anti-His antibody (Penta-His antibody; QIAGEN) (13).
Production and purification of EthA.
A recombinant His-tagged form of EthA was purified from M. smegmatis mc2155 overexpressing ethA from pVVethA. mc2155/pVVethA cells (3 g [wet weight]) resuspended in 3 ml of buffer A (25 mM Tris-HCl, pH 7.5, 300 mM NaCl) were disrupted by probe sonication for 8 min in the form of 60-s pulses with 90-s cooling intervals. The sonicate was centrifuged for 5 min at 2,000 × g at 4°C, and the supernatant from this centrifugation was loaded onto a BD TALON spin column (Clontech). Unbound proteins were removed by washing the resin with buffer A containing 10 mM imidazole. His-tagged EthA bound to the resin was then gradually eluted with buffer A containing 50 and 300 mM imidazole. Fractions containing approximately 90% pure EthA were combined, desalted by using a PD-10 column (Amersham Pharmacia Biotech), and concentrated using a Vivaspin 6 centrifugal concentrator (3-kDa molecular-weight cutoff) (Sartorius). The purified protein was immediately used in cell-free assays.
In vitro metabolism of ISO by purified EthA.
The in vitro activity of EthA on ISO was assayed as described by Vannelli and collaborators (25) with minor modifications. Briefly, the reaction mixture contained 100 mM NaCl, catalase (75 U ml−1), superoxide dismutase (75 U/ml), bovine serum albumin (0.1 mg/ml), a NADPH regenerating system consisting of glucose-6-phosphate dehydrogenase (2.5 U/ml), glucose-6-phosphate (25 mM), and NADPH (1 mM), 40 μg of purified EthA protein, ISO dissolved in 40 μl of DMSO (final concentration, 2 μg/ml), and 25 mM Tris-HCl buffer (pH 7.5) in a final volume of 4 ml. Reactions were carried out in pentaplicate. Reactions were stopped with 4 ml of chloroform after 60 min of incubation at 37°C and left rocking for 30 min at room temperature. Organic phases were removed, combined together, dried under a flow of nitrogen, and stored at −20°C. Immediately before liquid chromatography-mass spectrometry (LC/MS) analysis, samples were dissolved in DMSO and analyzed on an LC/MS apparatus (Agilent 1100 series) using a short C18 Zorbax column, a methanol-water gradient containing 0.07% of ammonium formate, and an atmospheric pressure electrospray source (positive mode).
Cell-free assays for mycolic acid and short-chain unsaturated fatty acid synthesis.
M. bovis BCG cells (1 g [wet weight]) resuspended in 25 mM Tris-HCl (pH 7.5) were disrupted by probe sonication as described above and centrifuged for 5 min at 2,000 × g, and the resulting sonicate was used as the enzyme source for in vitro synthesis of mycolates. Reaction mixtures contained M. bovis BCG sonicate (3 mg of proteins), 1 μCi of [1,2-14C]acetic acid (specific activity, 113 Ci/mol; MP Biomedicals, Inc.), partially purified EthA (up to 10 μg), ISO dissolved in 2.5 μl of DMSO (final concentrations, 0, 2, or 5 μg/ml), and 25 mM Tris-HCl buffer (pH 7.5) in a total volume of 250 μl. Reaction mixtures were preincubated on ice for 10 min and then incubated for 2 h at 37°C, with the reactions stopped by the addition of 1 ml of 15% tetrabutylammonium hydroxide (Aldrich). Mycolic acid methyl esters were then prepared as described previously (17) and analyzed by thin-layer chromatography (TLC) on silica gel 60-precoated F254 plates (E. Merck, Darmstadt, Germany) using n-hexane:ethyl acetate (95:5 by volume, three developments) as the eluent. TLC plates were exposed to Kodax Biomax MR films for 7 days at −70°C.
The in vitro effect of ISO and EthA activation on the activity of the Δ9 acyl-CoA desaturase DesA3 was monitored using a recombinant form of DesA3 purified from M. smegmatis as the enzyme source and M. smegmatis membranes as a source of cofactors for the desaturation system (18). For the production of DesA3, the desA3 gene was PCR amplified from M. tuberculosis H37Rv genomic DNA using primers desA3.jam1 (5′-CCCGGATCCATGGCGATCACTGACGTCGACG-3′) and desA3.jam2 (5′-CGTCTAGAGGCTGCCAGATCGTCGGGTTC-3′) and placed under control of the inducible acetamidase promoter in the mycobacterial expression plasmid pJAM2 (22). The resulting plasmid, pJAMdesA3, was electroporated into M. smegmatis mc2155, and the recombinant DesA3 protein was purified from mc2155/pJAMdesA3 cells following the same protocol as for EthA. Cell-free reaction mixtures contained 0.5 mg of M. smegmatis membrane proteins, 3 μg of partially purified DesA3, 6 μg of partially purified EthA, 500 μM palmitoyl-CoA, 1 mM NADPH, ISO dissolved in 3.2 μl of DMSO (final concentrations, 0 or 10 μg/ml), and 25 mM Tris-HCl buffer (pH 7.5) in a final volume of 320 μl. Reaction mixtures were incubated for 75 min at 30°C, and reactions were stopped with 2 ml of chloroform:methanol (2:1, by volume). The samples were left rocking for 30 min at room temperature and centrifuged, and organic phases were removed and dried. Fatty acid methyl esters were prepared with the 3 N methanolic HCl kit from Supelco at 80°C overnight and analyzed by gas chromatography on a Shimadzu GC-14A chromatograph using a methyl silicone 5% phenyl column operating at a temperature of 175°C for 2 min, followed by a programmed increase of 8°C/min to 300°C. The eluted peaks were identified by comparison of their retention time with those of fatty acid methyl ester standards (Supelco).
RESULTS AND DISCUSSION
Isolation and characterization of ISO-resistant mutants of M. tuberculosis.
With the goal of identifying the cellular targets of ISO in the tubercle bacillus, we have isolated spontaneous ISOr mutants of M. tuberculosis. Two strains of M. tuberculosis were used in the screening: the laboratory strain H37Rv and a mutT1 transposon mutant of M. tuberculosis Mt103 which we had shown earlier to display a hypermutator phenotype in the presence of rifampin or isoniazid (7). Mutants were selected at 37°C on 7H11 plates supplemented with OADC and 10 μg/ml ISO (four to five times the MIC of ISO against wild-type M. tuberculosis in this medium) and scored for ISO resistance 4 to 7 weeks postinoculation. Selection was performed in the presence of oleic acid in the medium (in the form of OADC) to avoid selecting ISOr mutants carrying mutations in the Δ9 acyl-CoA-desaturase DesA3, an already-known target of the drug (18). Twenty-one independent mutants were isolated from the parent H37Rv and MT1K strains at a frequency of 10−8 and 2 × 10−8, respectively. PCR amplification and sequencing of the promoter regions and coding sequences of the desA3, ethR, and ethA genes in these strains revealed that while none of them carried mutations in desA3 or ethR, two had undergone mutations within the coding sequence of ethA. The nucleotide deletion at position 1218 in strain ISO-R33 results in a frameshift mutation, while the three-nucleotide deletion at positions 164 to 166 of strain ISO-R22 results in a two-amino-acid change (SD→Y). It is noteworthy that the mutation identified in ISO-R22 is located between two highly conserved motifs (DxxxGxGxxG and FxGxxxHxxxWP) of FAD- and NADPH-dependent Baeyer-Villiger monooxygenases known to be critical for catalysis and that it more particularly affects an amino acid residue that is conserved among other flavin-containing monooxygenases (D56) (10, 26). Data thus suggested that the activity of EthA on ISO might be affected if not abolished in those two mutants. Both strains displayed a high level of ISO resistance, with MICs greater than 40 μg/ml (for solubility reasons, higher concentrations of ISO could not be tested) (Table 1). As expected, they also showed cross-resistance to ETH (MICs of >10 μg/ml, compared to an MIC of ∼5 μg/ml for the parent strains, as determined by the agar dilution method).
TABLE 1.
MICs of ISO against M. tuberculosis H37Rv and Mt103, the complemented and noncomplemented ISOr mutants, and M. tuberculosis H37Rv overexpressing ethA and ethR in 7H9 medium supplemented with ADC
| Strain | MIC of ISO (μg/ml) for strain with:
|
||
|---|---|---|---|
| pVV16 | pVVethA | pVVethR | |
| M. tuberculosis H37Rv | 5-10 | 2.5 | >40a |
| M. tuberculosis Mt103 | 5 | NDb | ND |
| M. tuberculosis MT1K ISO-R22 | >40a | 5-10 | ND |
| M. tuberculosis MT1K ISO-R33 | >40a | 5-10 | ND |
For solubility reasons, 40 μg/ml was the highest concentration of ISO that could be tested.
ND, not determined.
To determine whether ISO resistance in these strains arose from the mutations in ethA, a functional copy of the ethA gene carried by the multicopy vector pVVethA was introduced into each of the mutants and the MIC of ISO against the complemented mutant strains was determined. Complementation restored ISO susceptibility in both mutants to levels comparable to those of wild-type M. tuberculosis Mt103 and H37Rv (Table 1). The slight difference in drug sensitivity between H37Rv/pVVethA and the complemented mutants may be accounted for by the different background from which the mutants arise (the mutants were derived from the clinical isolate Mt103), the possible competition of the wild-type and mutated versions of EthA for ISO in the mutants, the total amount of active EthA produced by each strain, or the deleterious effect of coexpressing a mutated form and an active form of EthA on the oligomerization of this enzyme in the mutants (9). Nevertheless, it can be concluded from these results that ISO resistance in the two mutants is solely due to the reduced or complete lack of activity of EthA on ISO and that this monooxygenase is thus absolutely required for ISO to inhibit its lethal enzymatic target(s) in M. tuberculosis. Although the activation-dependent inhibitory effect of ISO on its cellular targets most likely explains this requirement, it is also possible that EthA serves to retain ISO or its metabolites inside the bacterial cell, as previously reported with ETH (12).
Effect of overexpressing ethA and ethR on susceptibility of M. tuberculosis to ISO.
Overexpression of ethA in M. tuberculosis H37Rv increased two- to fourfold the sensitivity of this strain to ISO, consistent with what had been reported earlier for M. bovis BCG (8) (Table 1). However, in striking contrast with earlier work which showed only a modest effect to no effect of the level of expression of ethR on the susceptibilities of M. bovis BCG, M. smegmatis, and M. tuberculosis to ISO (2, 8), we found that the overexpression of ethR from pVVethR in M. tuberculosis H37Rv resulted in more than an eightfold increase in resistance to the drug (Table 1). Differences in the mycobacterial species and strains used in the different studies and/or in the levels of expression of ethR in the various overexpressors may account for this apparent discrepancy.
In vitro metabolism of ISO by purified EthA.
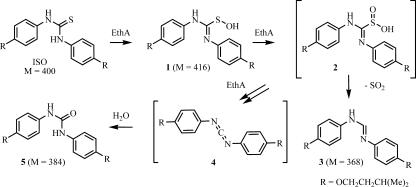
To establish whether EthA interacted with ISO directly and to identify the metabolites resulting from their interaction, we partially purified recombinant EthA from M. smegmatis overproducing a His-tagged version of this enzyme and incubated it with ISO as described in Materials and Methods. LC/MS analysis of the control experiment missing EthA revealed the presence of ISO (m/z = 401) along with trace amounts of an oxidized species (m/z = 417). LC/MS analyses of the reaction mixtures containing EthA clearly pointed out the occurrence of several compounds (Fig. 1). Aside from unreacted ISO, the most important signals detected were the following: (i) a species featuring a clear m/z signal at 385, compatible with the urea derivative (compound 5 in Fig. 1) (M = 384); (ii) a species featuring a clear m/z signal at 369, compatible with the formimidamide (compound 3 in Fig. 1) (M = 368). Lesser species were observed, especially two unresolved compounds featuring m/z signals at 417, compatible with oxidized forms of ISO (M = 416). Other m/z signals observed (m/z = 474 and 546) are less easily explained and may be experimental artifacts.
FIG. 1.
The proposed activation process of ISO.
The two other thio-bearing anti-TB drugs, ETH and TAC, were demonstrated to undergo EthA-catalyzed successive oxidations of their sulfur atom. These unstable oxidized intermediates are then further transformed into reactive species thought to be responsible for the antimycobacterial activity of these drugs. In vitro, the occurrence of such reactive species was demonstrated by the identification of end products characteristic of their chemical reactivity (6, 9, 12, 19, 25). In our case, the in vitro action of EthA on ISO led to the formation of the formimidamide (compound 3 in Fig. 1) as well as the urea derivative (compound 5). As depicted in Fig. 1, we suggest related transformations leading to these two compounds. Oxidation reactions of the sulfur atom of ISO would lead to the intermediate (compound 2), which would then undergo an extrusion reaction to give the formimidamide (compound 3). Further chemical transformations of intermediate (compound 2) (an EthA-based oxidation followed by an elimination) would lead to the reactive carbodiimide (compound 4), which, in our experimental setting, would be hydrolyzed into the urea derivative (compound 5).
In conclusion, LC/MS analysis of the reaction products resulting from the action of EthA on ISO in vitro revealed the occurrence of stable species arising from initial oxidation processes leading to reactive species. These results are in agreement with the previously reported occurrence of related reactive species following incubation of ETH or TAC with EthA and with the previously suggested inhibitory activity of these metabolites (6, 12, 19, 25).
Effect of ISO activation on the synthesis of mycolic acids and unsaturated short-chain fatty acids by mycobacterial cell extracts.
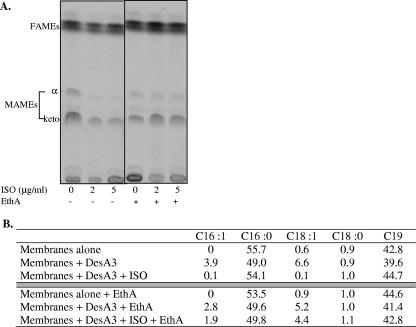
ISO inhibits the synthesis of mycolic acids through the inhibition of an as yet unidentified enzyme(s) and that of short-chain Δ9 monounsaturated fatty acids through the inhibition of the Δ9 desaturase DesA3 (17-18, 27). We were thus interested in determining whether the in vitro activation of ISO by EthA stimulated the inhibitory effect of the drug on these two metabolic pathways. To this end, two cell-free assays were designed. Mycolic acid synthesis was monitored using M. bovis BCG sonicates as an enzyme source and [1,2-14C]acetic acid as the radiolabeled substrate. The catalytic activity of DesA3 was measured in an assay containing a partially purified form of the DesA3 protein, palmitoyl-CoA, and M. smegmatis membranes as a further source of substrates and cofactors for the desaturation system. As expected, the addition of ISO to the first assay resulted in the inhibition of the synthesis of both alpha- and ketomycolates in M. bovis BCG extracts (Fig. 2A). Consistent with earlier observations, the addition of ISO to the second assay also completely inhibited the stimulatory effect of DesA3 on the in vitro synthesis of palmitoleic and oleic acids (Fig. 2B) (18). Unexpectedly, however, the addition of purified EthA to these assays almost totally abolished the inhibitory effect of the drug on mycolic acid, palmitoleic acid, and oleic acid synthesis (Fig. 2). We conclude from these experiments that the addition of purified EthA to the reaction mixtures probably pushed the transformation of ISO towards the formation of downstream inactive metabolites. Thus, as shown earlier for ETH (25) and supporting the above hypothesis of an active—perhaps carbodiimide—intermediate, it is likely that the active metabolite(s) of ISO is a transient and/or unstable intermediate(s) formed in the process of the oxidative transformation and decomposition of the drug.
FIG. 2.
Effect of EthA on the activity of ISO in cell-free assays. (A) Mycolic acid synthesis by M. bovis BCG cell extracts. The incorporation of [1,2-14C]acetate into the mycolic acids of M. bovis BCG sonicates incubated in the presence of different concentrations of ISO and in the presence (+) or absence (−) of partially purified EthA (10 μg) is shown. Mycolic acid methyl esters (MAMEs) and fatty acid methyl esters (FAMEs) were analyzed by TLC followed by autoradiography as described in Materials and Methods. (B) In vitro synthesis of unsaturated short-chain fatty acids. The activity of DesA3 was monitored in the presence or absence of ISO and EthA in a reaction containing M. smegmatis membranes, partially purified DesA3, palmitoyl-CoA, and NADPH. Fatty acids were extracted from the reaction mixtures, derivatized, and analyzed by gas chromatography. Their relative percentages in the assay mixtures are shown. C16:0, palmitic acid; C16:1, palmitoleic acid; C18:1, oleic acid; C18:0, stearic acid; C19, tuberculostearic acid.
Conclusions.
Through the selection of spontaneous ISOr mutants of M. tuberculosis and complementation studies, we have shown that EthA is absolutely required for ISO to exert its lethal effect on M. tuberculosis. Based on our biological data, the identification of two metabolites resulting from the in vitro action of EthA on ISO, and on what has been previously described for ETH and TAC, we thus conclude that ISO is a prodrug activated by oxidation reactions catalyzed by EthA. Among the reaction products, the corresponding carbodiimide is likely to arise from these oxidations, and we propose that this is the actual active form of ISO. As for ETH and TAC, it remains to be determined whether the reactive species derived from ISO act via covalent bond formation within the active site(s) of the enzymatic target(s) or through the formation of small adducts inside M. tuberculosis cells which would then become the actual inhibitors of these enzymes. Work is now in progress in our laboratories to characterize the remaining ISOr mutants of M. tuberculosis that were isolated in the course of this study and to identify the enzymatic targets of the drug.
Acknowledgments
This work was supported by the Institut Pasteur (GPH-Tuberculose) and National Institute of Allergy and Infectious Diseases/National Institutes of Health grant 5R01AI063054-01. J.K. is a recipient of a postdoctoral fellowship from the Heiser Program for Research in Leprosy and Tuberculosis (New York Community Trust).
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Anonymous. 1970. Discussion on isoxyl. Antibiot. Chemother. 16:187-202. [Google Scholar]
- 2.Baulard, A. R., J. C. Betts, J. Engohang-Ndong, S. Quan, R. A. McAdam, P. J. Brennan, C. Locht, and G. S. Besra. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 275:28326-28331. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy, F. D., and K. Ou. 1984. Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium. Tetrahedron Lett. 25:839-842. [Google Scholar]
- 4.Bhowruth, V., A. K. Brown, R. C. Reynolds, G. D. Coxon, S. P. Mackay, D. E. Minnikin, and G. S. Besra. 2006. Symmetrical and unsymmetrical analogues of isoxyl; active agents against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 16:4743-4747. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs worldwide, 2000-2004. Morb. Mortal. Wkly. Rep. 55:301-305. [PubMed] [Google Scholar]
- 6.DeBarber, A. E., K. Mdluli, M. Bosman, L.-G. Bekker, and C. E. Barry III. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dos Vultos, T., J. Blasquez, J. Rauzier, I. Matic, and B. Gicquel. 2006. Identification of nudix hydrolase family members with an antimutator role in Mycobacterium tuberculosis and Mycobacterium smegmatis. J. Bacteriol. 188:3159-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dover, L. G., A. Alahari, P. Gratraud, J. M. Gomes, V. Blowruth, R. C. Reynolds, G. S. Besra, and L. Kremer. 2007. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob. Agents Chemother. 51:1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraaije, M. W., N. M. Kamerbeek, A. J. Heidekamp, R. Fortin, and D. B. Janssen. 2004. The prodrug activator EtaA from Mycobacterium tuberculosis is a Baeyer-Villiger monooxygenase. J. Biol. Chem. 279:3354-3360. [DOI] [PubMed] [Google Scholar]
- 10.Fraaije, M. W., N. M. Kamerbeek, W. J. H. van Berkel, and D. B. Janssen. 2002. Identification of a Baeyer-Villiger monooxygenase sequence motif. FEBS Lett. 518:43-47. [DOI] [PubMed] [Google Scholar]
- 11.Global Alliance for TB Drug Development. 2001. Tuberculosis. Scientific blueprint for TB drug development. Tuberculosis 81(Suppl. 1):1-52. [DOI] [PubMed] [Google Scholar]
- 12.Hanoulle, X., J.-M. Wieruszeski, P. Rousselot-Pailley, I. Landrieu, C. Locht, G. Lippens, and A. Baulard. 2006. Selective intracellular accumulation of the major metabolite issued from the activation of the prodrug ethionamide in mycobacteria. J. Antimicrob. Chemother. 58:768-772. [DOI] [PubMed] [Google Scholar]
- 13.Korduláková, J., M. Gilleron, K. Mikusová, G. Puzo, P. J. Brennan, B. Gicquel, and M. Jackson. 2002. Definition of the first mannosylation step in phosphatidylinositol synthesis: PimA is essential for growth of mycobacteria. J. Biol. Chem. 277:31335-31344. [DOI] [PubMed] [Google Scholar]
- 14.Lambelin, G. 1970. Pharmacology and toxicology of isoxyl. Antibiot. Chemother. 16:84-95. [DOI] [PubMed] [Google Scholar]
- 15.Martin, A., M. Camacho, F. Portaels, and J.-C. Palomino. 2003. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob. Agents Chemother. 47:3616-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. B. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, and P. Nunn. 1998. Global surveillance for antituberculosis-drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. [DOI] [PubMed] [Google Scholar]
- 17.Phetsuksiri, B., A. R. Baulard, A. Cooper, D. E. Minnikin, J. D. Douglas, G. S. Besra, and P. J. Brennan. 1999. Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrob. Agents Chemother. 43:1042-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phetsuksiri, B., M. Jackson, H. Scherman, M. R. McNeil, G. S. Besra, A. R. Baulard, R. A. Slayden, A. E. DeBarber, C. E. Barry III, M. S. Baird, D. C. Crick, and P. J. Brennan. 2003. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 278:53123-53130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian, L., and P. R. Ortiz de Montellano. 2006. Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem. Res. Toxicol. 19:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivanandan, K., B. S. Sandanaraj, and S. Thayumanavan. 2004. Sequences in dendrons and dendrimers. J. Org. Chem. 69:2937-2944. [DOI] [PubMed] [Google Scholar]
- 21.Titscher, R. 1966. Monotherapie mit isoxyl/DAT bei tuberculose-asylierungsfallen. Prax. Pneumol. 20:202-206. [PubMed] [Google Scholar]
- 22.Triccas, J. A., T. Parish, W. J. Britton, and B. Gicquel. 1998. An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol. Lett. 167:151-156. [DOI] [PubMed] [Google Scholar]
- 23.Urbancik, B. 1966. A clinical trial of thiocarlide (4-4′ diisoamyloxythiocarbanilide). Tubercle 47:283-288. [DOI] [PubMed] [Google Scholar]
- 24.Urbancik, B. 1970. Clinical experience with thiocarlide (isoxyl). Antibiot. Chemother. 16:117-123. [DOI] [PubMed] [Google Scholar]
- 25.Vannelli, T. A., A. Dykman, and P. R. Ortiz de Montellano. 2002. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J. Biol. Chem. 277:12824-12829. [DOI] [PubMed] [Google Scholar]
- 26.Willetts, A. 1997. Structural studies and synthetic applications of Baeyer-Villiger monooxygenases. Trends Biotechnol. 2:55-62. [DOI] [PubMed] [Google Scholar]
- 27.Winder, F. G., P. B. Collins, and D. Whelan. 1971. Effects of ethionamide and isoxyl on mycolic acid synthesis in Mycobacterium tuberculosis BCG. J. Gen. Microbiol. 66:379-380. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2006. Tuberculosis. Fact sheet no. 104. http://www.who.int/mediacentre/factsheets/fs104/en/print.html.