Abstract
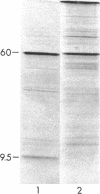
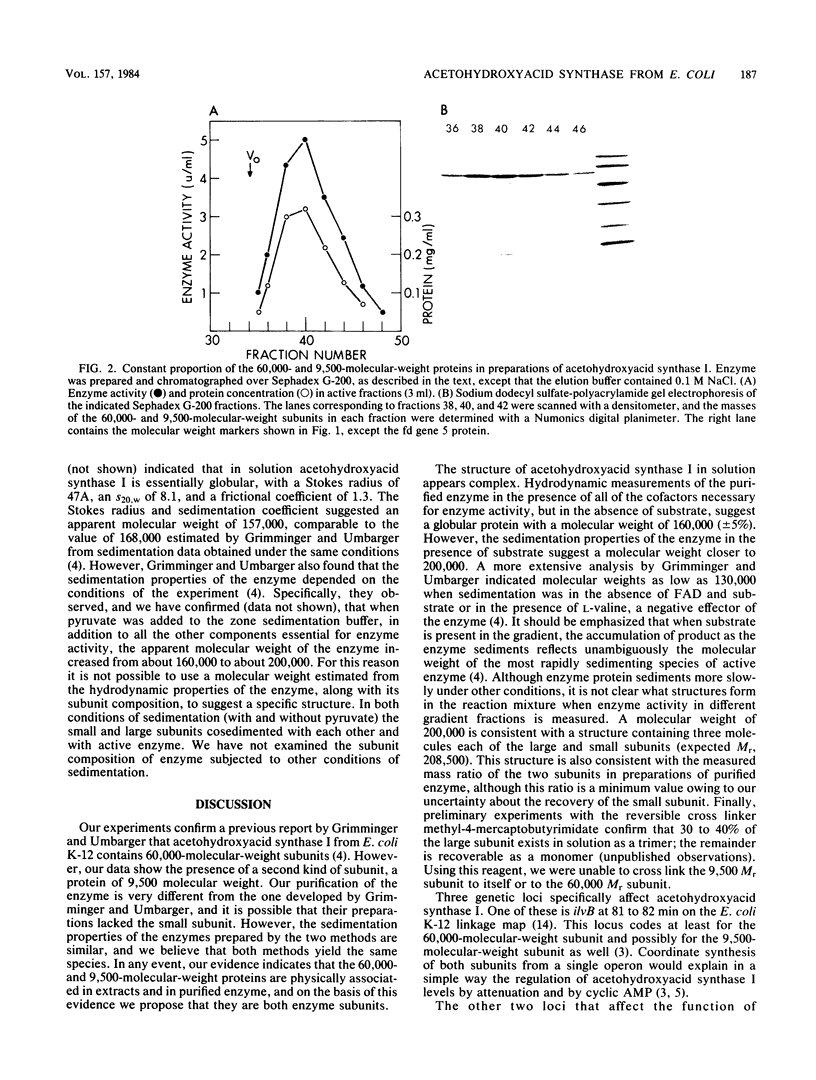
Acetohydroxyacid synthase I from Escherichia coli K-12 has been purified to near homogeneity. Analysis of the purified enzyme by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed the presence of two polypeptides, one with a molecular weight of 60,000 and one with a molecular weight of 9,500. These two polypeptides were present in constant proportion to each other and to enzyme activity. The molar ratio of the two polypeptides (Mr 9,500:60,000), estimated from stained polyacrylamide gels, was 1. Antisera raised against the 60,000 Mr polypeptide precipitated both the 60,000 and the 9,500 Mr polypeptides from extracts of cells labeled with [35S]methionine. The addition of sodium dodecyl sulfate before immunoprecipitation eliminated the smaller polypeptide, and only the larger one was recovered. The hydrodynamic properties of the native enzyme confirmed a previous report that the largest enzymatically active species has a molecular weight of about 200,000; this species contains both the 60,000- and 9,500-molecular-weight polypeptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Protein subunits: a table (revised edition). Arch Biochem Biophys. 1972 Mar;149(1):1–14. doi: 10.1016/0003-9861(72)90293-7. [DOI] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino M., Guardiola J., De Felice M., Favre R. Regulation of isoleucine and valine biosynthesis. Curr Top Cell Regul. 1978;14:29–73. doi: 10.1016/b978-0-12-152814-0.50006-x. [DOI] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J., Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J Bacteriol. 1980 Oct;144(1):68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T. C., Levinthal M. A new map location for the ilvB locus of Escherichia coli. Genetics. 1980 Sep;96(1):59–77. doi: 10.1093/genetics/96.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T., Friden P., Sutton A., Freundlich M. Cloning and expression of the ilvB gene of Escherichia coli K-12. Mol Gen Genet. 1982;186(3):378–384. doi: 10.1007/BF00729457. [DOI] [PubMed] [Google Scholar]
- Robinson C. L., Jackson J. H. New acetohydroxy acid synthase activity from mutational activation of a cryptic gene in Escherichia coli K-12. Mol Gen Genet. 1982;186(2):240–246. doi: 10.1007/BF00331856. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Lago C. T., Calvo J. M. IlvHI locus of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1054–1063. doi: 10.1128/jb.154.3.1054-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Newman T., McEwen J., Silverman P. M., Freundlich M. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J Bacteriol. 1982 Aug;151(2):976–982. doi: 10.1128/jb.151.2.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]