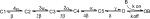Abstract
Resurgent tail Na+ currents were first discovered in cerebellar Purkinje neurons. A recent study showed that a 14-mer fragment of a mouse β4 subunit, β4154–167, acts as an intracellular open-channel blocker and elicits resurgent currents in Purkinje neurons (Grieco, T.M., J.D. Malhotra, C. Chen, L.L. Isom, and I.M. Raman. 2005. Neuron. 45:233–244). To explore these phenotypes in vitro, we characterized β4154–167 actions in inactivation-deficient cardiac hNav1.5 Na+ channels expressed in human embryonic kidney 293t cells. Intracellular β4154–167 from 25–250 μM elicited a conspicuous time-dependent block of inactivation-deficient Na+ currents at 50 mV in a concentration-dependent manner. On and off rates for β4154–167 binding were estimated at 10.1 μM−1s−1 and 49.1 s−1, respectively. Upon repolarization, large tail currents emerged with a slight delay at −140 mV, probably as a result of the rapid unblocking of β4154–167. Near the activation threshold (approximately −70 mV), resurgent tail currents were robust and long lasting. Likewise, β4154–167 induces resurgent currents in wild-type hNav1.5 Na+ channels, although to a lesser extent. The inactivation peptide acetyl-KIFMK-amide not only restored the fast inactivation phenotype in hNav1.5 inactivation-deficient Na+ channels but also elicited robust resurgent currents. When modified by batrachotoxin (BTX), wild-type hNav1.5 Na+ channels opened persistently but became resistant to β4154–167 and acetyl-KIFMK-amide block. Finally, a lysine substitution of a phenylalanine residue at D4S6, F1760, which forms a part of receptors for local anesthetics and BTX, rendered cardiac Na+ channels resistant to β4154–167. Together, our in vitro studies identify a putative S6-binding site for β4154–167 within the inner cavity of hNav1.5 Na+ channels. Such an S6 receptor readily explains (1) why β4154–167 gains access to its receptor as an open-channel blocker, (2), why bound β4154–167 briefly prevents the activation gate from closing by a “foot-in-the-door” mechanism during deactivation, (3) why BTX inhibits β4154–167 binding by physical exclusion, and (4) why a lysine substitution of residue F1760 eliminates β4154–167 binding.
INTRODUCTION
Mammalian voltage-gated Na+ channels are responsible for the generation of action potentials in excitable membranes. These channels consist of one large α subunit along with one or two small auxiliary β subunits (Catterall, 2000). Nine α subunits (Nav1.1–1.9) and four β subunits (β1–4) have been identified to date. When expressed in human embryonic kidney 293 (HEK293) cells, the α subunit alone forms a functional channel, which appears comparable with the native Na+ channel (Ukomadu et al., 1992).
Voltage-gated Na+ channels are activated by depolarization and, once open, are quickly inactivated through the action of an inactivation particle, which rapidly blocks the open pore (Armstrong and Benzanilla, 1977). Armstrong (1981) proposed a “foot-in-the-door” model for fast inactivation. His model states that (1) an inactivating particle can block the channel only if the activation gate is open and that (2) closing of the activation gate is hindered by a foot-in-the-door effect once the inactivation particle is in the channel mouth. Because Na+ channels do not leak during recovery from inactivation, the foot-in-the-door model also requires that the activation gate (door) is partially closed before the withdrawal of the inactivation particle (foot). The partially deactivated Na+ channel does not conduct while the inactivation particle is withdrawn from the pore. The inactivation particle likely contains an IFM motif located within the intracellular D3-D4 linker of the Na+ channel α subunit as deduced by site-directed mutagenesis (West et al., 1992). A synthetic peptide, acetyl-KIFMK-amide, can restore fast inactivation in inactivation-deficient rNav1.2 and hNav1.5 Na+ channels (Eaholtz et al., 1994; Tang et al., 1996; Wang and Wang, 2005).
In the cerebellum, Purkinje neurons often fire high-frequency action potentials with a rate near 100 Hz. Such rapid discharges require short refractory periods. Earlier classical studies indicated that Purkinje Na+ channels may be subject to a rapid, voltage-dependent open-channel block by an unknown endogenous blocker (Raman and Bean, 1997; Raman et al., 1997). By blocking the pore during depolarization and rapid unblocking upon repolarization, this endogenous blocker prevents the conventional fast inactivation particle from binding, thereby accelerating recovery. While the blocker is expelled from the pore, a brief resurgent Na+ current emerges upon repolarization. This resurgent tail Na+ current could account for the high frequency firings in Purkinje neurons (Raman and Bean, 2001). More recently, Grieco et al. (2005) reported that β4154–167, the cytoplasmic tail of the mouse β4 subunit (KKLITFILKKTREK), blocked Na+ channels and elicited resurgent Na+ currents in Purkinje neurons. These authors proposed that the β4 cytoplasmic tail is the endogenous open-channel blocker responsible for resurgent kinetics. The major Na+ channel isoform in cerebellar Purkinje neurons is the Nav1.6. However, resurgent Na+ current is not completely abolished in Nav1.6-null Purkinje neurons (Grieco and Raman, 2004), indicating that the resurgent Na+ current may not be limited to neuronal Nav1.6.
The β4 subunit has been detected in cardiac tissues (Yu et al., 2003; Maier et al., 2004), but its role in the heart remains unknown. Most, if not all, studies concerning resurgent currents have used neurons from the central nervous system or dorsal root ganglion (Do and Bean, 2004; Grieco and Raman, 2004; Cummins et al., 2005). In this study, we asked whether mouse β4154–167 acts as an open-channel blocker in cardiac hNav1.5 channels. We addressed this question primarily using inactivation-deficient mutant hNav1.5-L409C/A410W Na+ channels (hNav1.5-CW) expressed in HEK293t cells. In this mutant channel, the native D1S6 residues leucine (L) at position 409 and alanine (A) at position 410 were replaced by cysteine (C) and tryptophan (W), respectively. This D1S6 region is not directly involved in acetyl-KIFMK-amide binding, although mutations at this site may prevent access of the intrinsic fast inactivation particle to the inner cavity (Wang and Wang, 2005). The HEK expression system allowed us to characterize pharmacological actions of β4154–167 and to define the β4154–167 receptor in the hNav1.5 channel by site-directed mutagenesis. Our results suggest that β4154–167 binds near the activation gate within the inner cavity and briefly prevents the gate from closing, which is analogous to the foot-in-the-door mechanism proposed by Armstrong (1981).
MATERIALS AND METHODS
Site-Directed Mutagenesis
We used the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene) to create an inactivation-deficient hNav1.5-CW mutant clone within the pcDNA1/Amp vector (Wang et al., 2003; Wang and Wang, 2005). An additional mutation, hNav1.5-CW/F1760K, was derived using the same method. Other inactivation-deficient mutants, rNav1.4-L435W/L437C/A438W (rNav1.4-WCW) and rNav1.4-WCW/F1579K (Wang et al., 2003), were also created.
Transient Transfection
HEK293t cells were grown to ∼50% confluence in Dulbecco's modified Eagle's medium (Life Technologies) containing 10% FBS (HyClone), 1% penicillin and streptomycin solution (Sigma-Aldrich), 3 mM taurine, and 25 mM HEPES (Life Technologies) and were transfected by calcium phosphate precipitation. Transfection of 5–10 μg of wild-type hNav1.5-CW along with 10–20 μg of rat β1-pcDNA1/Amp and 1 μg of reporter CD8-pih3m was adequate for current recording. Rat β1 subunit was included to increase the level of the channel expression. Cells were replated 15 h after transfection in 35-mm dishes, maintained at 37°C in a 5% CO2 incubator, and used after 1–4 d. Transfection-positive cells were identified with immunobeads (CD8-Dynabeads; Invitrogen).
Whole-Cell Voltage Clamp
Whole-cell configuration was used to record Na+ current (Hamill et al., 1981). Borosilicate micropipettes were pulled (P-87; Sutter Instrument Co.) and heat polished. Pipette electrodes contained 100 mM NaF, 30 mM NaCl, 10 mM EGTA, and 10 mM HEPES adjusted to pH 7.2 with CsOH. The pipette electrodes had a tip resistance of 0.5–1.0 MΩ. Access resistance was generally 1–2 MΩ. We minimized the series resistance (>85%) and corrected the leak and capacitance using a patch clamp amplifier (Axopatch 200B; Molecular Devices). Holding potential was set at −140 mV. Further correction was achieved by P/−4 protocol. The tail current was normally recorded at a rate of 20–40 μs per point. It is noteworthy that the accuracy of the rising phase of the tail current records is dependent on the speed of the Axopatch amplifier. It could take ∼170 μs to reach 80% of the final voltage applied (Cota and Armstrong, 1989). With supercharging compensation by Axopatch, the tail currents appeared to reach their peak amplitude more rapidly. Unfortunately, a large spike was introduced by this device and prevented us from using such correction. To verify that the differences existed in tail currents with and without the peptide, we superimposed the tail currents for direct comparison and included the measurement of the area under the tail currents (nano-amperes × milliseconds).
Experiments were performed at room temperature (22–24°C) under a Na+-containing bath solution with 65 mM NaCl, 85 mM choline Cl, 2 mM CaCl2, and 10 mM HEPES adjusted to pH 7.4 with tetramethylammonium hydroxide. In a few cases, we exchanged NaCl with choline Cl with a final external Na+ concentration of 0 or 130 mM. An unpaired t test was used to evaluate estimated parameters (mean ± SEM or fitted value ± SEM of the fit); P values of < 0.05 were considered statistically significant.
Drug Solutions
Peptide β4154–167 with a mouse β4 sequence (KKLITFILKKTREK) was synthesized by Biopeptide Co. (>95% pure), dissolved in the pipette solution at a 5-mM stock concentration, aliquoted, and stored at −20°C. The final peptide solution was prepared, stored at −20°C, and thawed. We used a 1-ml syringe to withdraw an appropriate amount of the final peptide solution and kept it at 4°C. Under these conditions, we observed no changes in β4154–167 activity. Under whole-cell configuration, HEK293t cells survived routinely for >1 h, which is long enough for the peptide block to reach its steady state even at low concentrations. Acetyl-KIFMK-amide was synthesized, dissolved at a 10-mM stock concentration, and prepared as described previously (Wang and Wang, 2005). Batrachotoxin (BTX) was provided by J. Daly (National Institutes of Health, Bethesda, MD) and was dissolved in DMSO at 0.5 mM. The final concentration of BTX was 4 μM.
Computer Simulation
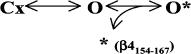
For β4154–167 block, we used a simplified gating model from Raman and Bean (2001) without the fast inactivation particle.This is a statistical model in which channels must transition through five sequential closed states (C1–C5) before reaching the open state (O), which allows Na+ ions to flow through. First-order kinetics describe these transitions using the voltage-dependent coefficients α, β, γ, and δ (in seconds−1), which are: α = 1.6 eV/40, β = 0.3 e−V/40, γ = 50 eV/100, and δ = 0.6 e−V/25, where V is in millivolts. These coefficients were obtained by repetitive approximation to minimize the mean square error between the experimental data and the simulated current traces. The open channel is blocked by peptide β4154–167 (B) as governed by kon (Molar−1 × seconds−1) and koff (seconds−1).
(SCHEME 1).
RESULTS
Block of Inactivation-Deficient Mutant Na+ Currents by Internal β4154–167
We investigated the effects of β4154–167 first in inactivation-deficient hNav1.5-L409C/A410W mutant Na+ channels at 50 mV where fast inactivation was minimal or absent (Fig. 1 A). After the pipette break-in, the outward current became larger initially as the pipette solution contained a high [Na+]in (65 mM). Within a few minutes, the Na+ currents reached a quasi steady-state level, and, from then on, β4154–167 began to block the maintained Na+ currents as if β4154–167 restored the fast inactivation phenotype (Fig. 1 B). With 125 μM β4154–167 in the pipette, this time-dependent blocking phenotype reached its steady state by ∼15–30 min. At steady state, the current decay was well fitted by a single exponential function with a time constant (τ) of 0.84 ± 0.18 ms (n = 5; Fig. 1 C, dashed line). Such a time-dependent block phenotype was detected in dissociated neurons with the help of a wasp toxin, β-pompilidotoxin, which slowed the fast inactivation gating considerably (Grieco and Raman, 2004). In contrast, without peptide present, most hNav1.5-CW Na+ currents were noninactivating at 50 mV (Fig. 1 A; Wang and Wang, 2005).
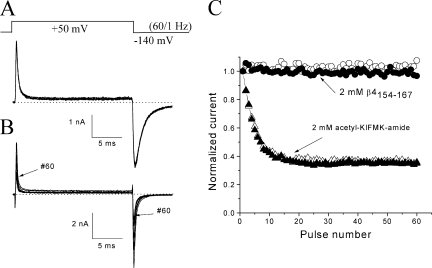
Figure 1.
Time-dependent block induced by β4154–167 at 50 mV. Current traces of hNav1.5-CW mutant channels were recorded after the pipette break-in under the whole-cell configuration. The pipette solution contained 125 μM β4154–167. (A) Currents became larger during initial dialysis but exhibited little or no fast inactivation. Traces were recorded at the times labeled. (B) The time-dependent block was progressively developed during continued dialysis and reached steady state around 15–30 minutes. (C) The time-dependent block was well fitted by a single exponential function with a τ value of 0.86 ms (dashed line). Holding potential was set at −140 mV.
On and Off Rates of β4154–167 Binding in Inactivation-Deficient Na+ Channels
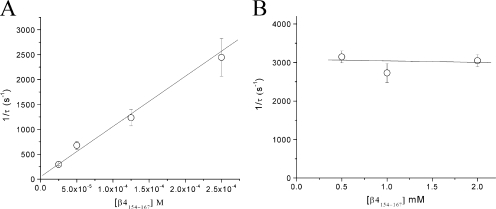
To determine the on and off rates of β4154–167 block, we measured the τ values at various peptide concentrations ranging from 25 to 250 μM as described in Fig. 1 C. The τ values were inverted and plotted against the concentration (Fig. 2 A; n = 5). The data were fitted by a linear regression and yielded on and off rate values of 10.1 μM−1s−1 and 49.1 s−1, respectively. The calculated equilibrium dissociation constant (KD = koff/kon) was 4.9 μM. For comparison, the estimated values for the ShB peptide in Shaker K+ channels at 50 mV are 4.7 μM−1s−1, 13.8 s−1, and 2.9 μM for kon, koff, and KD, respectively (Murrell-Lagnado and Aldrich, 1993), or approximately two, four, and two times smaller than those of β4154–167 in hNav1.5-CW Na+ channels.
Figure 2.
On and off rates of β4154–167 binding at 50 mV. (A) The τ values were recorded in inactivation-deficient hNav1.5-CW mutant channels at β4154–167 concentrations ranging from 25 to 250 μM, inverted, and plotted against the corresponding concentration (n = 5). The plot was fitted by a linear regression to yield y = 49.1 + (10.1 × 106) × x. Off rate (k-1 = x intercept) was estimated to be 49.1 s−1, and the on rate (k1 = slope) was 10.1 [μM]−1s−1. (B) The τ values were recorded at β4154–167 concentrations ranging from 0.5 to 2 mM, inverted, and plotted against the corresponding concentration. The τ values did not vary significantly at this concentration range (P > 0.05; n = 6). Error bars represent SEM.
At 0.5 mM β4154–167, the decay of the current has a time constant of ∼0.3 ms, which overlapped the activation time course. The half-time to reach the peak amplitude shown in Fig. 1 A is ∼0.33 ms. Fig. 2 B shows that at the concentration ranging from 0.5 to 2 mM, 1/τ values were nearly constant at ∼3,000 s−1. This phenotype implies that when the peptide concentration is ≥0.5 mM, channel activation limits the on rate of the β4154–167 block. Mathematical simulation of this phenomenon will be given in the Discussion.
Voltage Dependence of the Time-Dependent Block by β4154–167
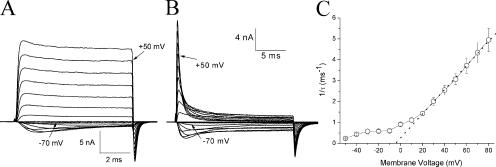
The family of hNav1.5-CW Na+ currents at various voltages in the absence (Fig. 3 A) and presence of β4154–167 peptide (Fig. 3 B) showed an apparent voltage dependence of the time-dependent block by 125 μM β4154–167, particularly at voltages ≥20 mV. This voltage-dependent phenotype was found at all β4154–167 concentrations (25 μM–2 mM). The time-dependent block of these currents could be fitted by a single exponential function from −50 to 80 mV. The time constant was inverted (1/τ) and plotted against voltage. Fig. 3 C shows that the value of 1/τ is strongly voltage dependent from 20 to 80 mV in the presence of 1 mM β4154–167. At this voltage range, the relationship is linear (Fig. 3 C, dotted line): the larger the voltages, the faster the time constants. At voltage <10 mV, the time-dependent block deviates from the dotted line (Fig. 3 C) and becomes less voltage dependent. Control currents without peptide indicated that minimal inactivation occurred at voltages greater than −10 mV. However, a greater residual fast inactivation appeared in −50 to −20 mV (Fig. 3 A; Wang and Wang, 2005). The reason for this phenotype in control traces at voltages less than −20 mV is not clear. Thus, the time-dependent block may be even less than what we observed at less than −20 mV in Fig. 3 (B and C) because of the presence of residual fast inactivation. We conclude that under our experimental conditions, the time-dependent block of the open Na+ channel becomes progressively stronger when the voltage is >20 mV but is weak at <10 mV.
Figure 3.
Time-dependent block at various voltages. Families of superimposed hNav1.5-CW current traces were recorded at various voltages in the absence (A) and presence (B) of 125 μM β4154–167. Traces corresponding to −70 and 50 mV are labeled. For the steady-state block of β4154–167, a slower time frame was used in B, which was recorded ∼30 min after the pipette break-in. (C) The decaying phase of the current traces at 1 mM β4154–167 was fitted by a single exponential function with a time constant, τ. The τ value was inverted and plotted against the corresponding voltage (n = 6 except at −10 mV, where n = 3). Error bars represent SEM.
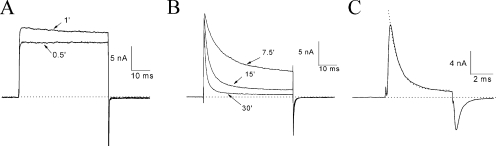
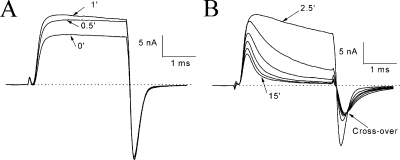
Altered Tail Currents at −140 mV in the Presence of β4154–167
In current traces shown in Figs. 1 and 3, tail currents seemed to decay slower in the presence of 125 μM β4154–167 (Fig. 1, A vs. B; and Fig. 3, A vs. B). A fast time frame, however, is needed to clearly resolve tail current kinetics. After the pipette break-in, outward inactivation-deficient Na+ currents at a test pulse of 50 mV again increased during the initial dialysis (Fig. 4 A). At the end of the test pulse, these outward currents became inward when the membrane voltage was switched to −140 mV. During the rapid deactivation of the open Na+ channel (Fig. 4 A), these inward tail currents decayed rapidly, which was well fitted by a single exponential function with a τ value of 144 μs. This τ value was similar to that without peptide present (τ = 132 ± 4 μs; n = 5). Although the time-dependent block by β4 peptide at 1 mM continued to develop during the test pulse, both the decay of the tail current and its peak amplitude were also progressively slowed and reduced, resulting in a crossover phenotype (Fig. 4 B; τ = 448 μs for 15-min trace).
Figure 4.
Altered tail currents by 1 mM β4154–167 after pipette break-in. (A) Initial run-up of inactivation-deficient Na+ currents at 50 mV under the whole-cell configuration. This phenomenon was the same as shown in Fig. 1 A except with a faster time frame. The tail currents decayed rapidly with a τ value of 144 μs. (B) As the time-dependent block continued to develop during the test pulse, the tail current also decayed more slowly. The arrow shows the tail current traces crossing over.
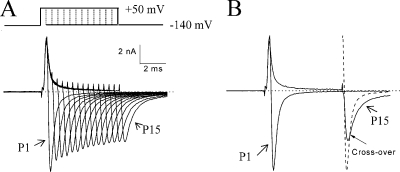
If the slowed tail current is caused by the unbinding of β4 peptide upon repolarization (Scheme 2), varying the duration of the test pulse (which enhances the binding of β4 peptide) should affect the tail current kinetics.Therefore, we used such a pulse protocol and measured their corresponding tail current in the presence of 1 mM β4154–167 (Fig. 5 A). The decay of the tail currents and the area under the current trace (3 ms) were measured. Superimposed tail currents (Fig. 5 B) show clear differences in tail current kinetics. The τ for trace 1 was 328 μs (area under the trace = 4.784 nA × ms), whereas the τ value for trace 15 was 667 μs (area under trace = 6.393 nA × ms). The τP15/τP1 ratio of tail currents was 1.98 ± 0.02 (n = 5). This result indicates that as more Na+ currents are blocked (O→O* in forward reaction; Scheme 2) during the test pulse, their tail currents become slower in kinetics (O*→O→C in backward reaction). Thus, the O* complex produced two apparent phenotypes on the tail current kinetics: it delayed the time to reach its peak, and it slowed the decay of the tail current. The first phenotype suggests that the activation gate cannot close the pore rapidly when the peptide is bound. The second phenotype suggests that a small fraction of O* continues to dissociate even after the tail reaches its peak. The effects on inactivation-deficient mutant Na+ currents by β4154–167 peptide did not appear to be isoform specific. Similar tail current phenotypes were observed in the rat skeletal muscle inactivation-deficient rNav1.4-WCW mutant channels (unpublished data).
(SCHEME 2).
Figure 5.
Tail current kinetics versus the duration of the test pulse. (A) Superimposed Na+ currents were recorded using a test pulse protocol with duration varying from 0.4 (trace P1) to 6 ms (trace P15) with a 0.4-ms step (top). The pipette solution contained 1 mM β4154–167. The τ values of tail currents were measured at 327 ± 18 μs and 649 ± 33 μs for traces P1 and P15, respectively (n = 5). (B) Traces P1 and P15 recorded from the first pulse and from the 15th pulse were compared side by side. Although most of the outward currents in trace P15 were blocked by β4154–167, large tail currents emerged after the test pulse. The dashed line shows that the tail current from trace P1 reached its peak earlier and decayed faster than that from trace P15. The arrow shows the two traces crossing over. The area under the tail current for trace 15 was larger than that for trace 1. Holding potential was set at −140 mV.
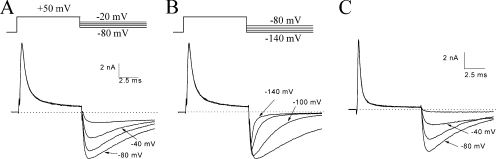
Resurgent Na+ Currents Near the Activation Threshold
The altered kinetics in tail currents suggests that the activation gate may not be able to close the channel rapidly (deactivation) when the peptide is bound. If this is true, what conditions will favor the appearance of resurgent tail Na+ currents upon repolarization? Scheme 2 shows that depolarization favors the forward reaction to reach O and eventually to form the O* complex, which results in the time-dependent block. Repolarization to −140 mV then favors the dissociation of the O* complex as well as the normal deactivation (fast tail currents; O→C). At the activation threshold, however, O↔C transition will be generally slow and reversible. If dissociation of the O* complex remains fast, we should observe robust resurgent currents at voltages near the activation threshold. Therefore, with this reasoning, we measured the resurgent Na+ currents at various membrane voltages. After the time-dependent block by 125 μM β4154–167 developed fully during the test pulse, a large resurgent current emerged when the voltage was switched to −80 mV (Fig. 6 A). The current increased rapidly to reach its peak and then decayed slowly (τ = 10.8 ± 2.2 ms; n = 6). This slow decay of the resurgent Na+ current could be partly attributed to the slow and reversible closing (deactivation) of the open Na+ channels (O↔C transition) at −80 mV. The rapid rising phase (τ = 232 ± 20 μs; n = 6), on the other hand, is mostly caused by the rapid dissociation of the peptide from its binding site. Fig. 6 B shows the resurgent currents from −140 to −80 mV. The rising phase of resurgent tail Na+ currents was not strongly voltage dependent. Therefore, the rising phases of resurgent tail Na+ currents are likely governed by the rapid unbinding of β4154–167 peptide. The peptide does not bind strongly with the inactivation-deficient Na+ channel effectively at voltages less than −20 mV, as indicated by its weak time-dependent block (Fig. 3 B) and by the peptide-induced large resurgent tail current (Fig. 6, A and B).
Figure 6.
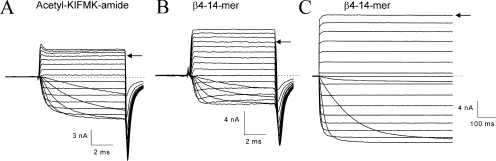
Resurgent Na+ currents near the activation threshold. (A) Tail currents were recorded at voltages ranging from −80 to −20 mV with a 20-mV step (top) in inactivation-deficient hNav1.5-CW mutant channels and were superimposed. The pipette contained 125 μM β4154–167. Resurgent Na+ currents were robust and long lasting with a τ value of 11.5 ms (10.8 ± 2.2 ms; n = 6) at −80 mV. (B) Tail currents were recorded in the same cell as shown in A at voltages ranging from −140 to −80 mV with a 20-mV step (top) and were superimposed. The decay of the tail currents was highly dependent on this voltage range. (C) Robust and long-lasting resurgent currents were detected near the activation threshold with the pipette containing 2 mM acetyl-KIFMK-amide. The pulse protocol was the same as shown in A (top). This experiment was repeated in five cells with similar results.
We also tested whether the inactivation peptide acetyl-KIFMK-amide can induce resurgent Na+ currents. As of yet, there is no report indicating that acetyl-KIFMK-amide generates resurgent tail currents (Eaholtz et al., 1994, 1999; Tang et al., 1996; Grieco et al., 2005). Fig. 6 C shows that acetyl-KIFMK-amide at 2 mM induces large resurgent tail Na+ currents comparable with those described in Fig. 6 A with β4154–167. This phenomenon is interesting because it implies that the Na+ channel with the weakened fast inactivation particle, such as in many genetic diseases (Cannon, 1996), could, in theory, also induce resurgent Na+ currents under appropriate conditions.
Lack of the Use-Dependent Block by β4154–167 Peptide
To investigate the use-dependent block of β4154–167, we applied repetitive pulses at 50 mV for 20 ms at 1 Hz and recorded Na+ currents (Fig. 7 A). Both the peak outward currents and peak inward tail currents were measured, normalized, and plotted against the pulse number (Fig. 7 C; open and closed circles, respectively). We found little additional use-dependent block of both the peak and tail currents. The decay of the tail currents was well fitted by an exponential decay with τ = 1.08 ± 0.11 ms (n = 6; measured at pulse 1). Under identical conditions, the inactivation peptide acetyl-KIFMK-amide produced additional use-dependent block on the outward peak as well as the inward tail currents by ∼65% (Fig. 7, B and C; open and closed triangles). This use-dependent phenotype for acetyl-KIFMK-amide in hNav1.5-CW inactivation-deficient mutant Na+ channels has been reported previously (Wang and Wang, 2005) and is clearly different from that of β4154–167. To account for such a use-dependent phenomenon, Eaholtz et al. (1999) suggested that there is a second binding site for acetyl-KIFMK-amide in the pore. By this comparison, there appears to be no second binding site for β4154–167 peptide within the pore. Interestingly, the tail currents induced by acetyl-KIFMK-amide decayed with a τ value of 0.27 ± 0.02 ms (n = 5), or four times faster than that of β4154–167.
Figure 7.
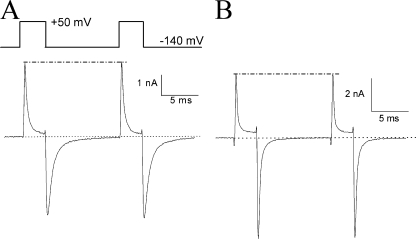
Use-dependent block by β4154–167 versus by acetyl-KIFMK-amide. (A) Superimposed currents were recorded during repetitive pulse (top; 50 mV/20 ms at 1 Hz). Current traces corresponded to the pulse numbers 1, 3, 5, 10, 20, 30, 40, 50, and 60. The pipette solution contained 2 mM β4154–167. (B) Superimposed currents were recorded under an identical protocol except the pipette solution contained 2 mM acetyl-KIFMK-amide. (C) Peak outward Na+ currents (open symbols) and peak inward tail currents (closed symbols) as shown in A and B were measured, normalized with respect to the peak value obtained from pulse 1, and plotted against the corresponding pulse number. Open and closed circles represent data from A in the presence of 2 mM β4154–167, whereas open and closed triangles are data from B with 2 mM acetyl-KIFMK-amide. Experiments were repeated in five separate cells with similar results. Use-dependent block of peak currents was minimal in the presence of 2 mM β4154–167 (2.9 ± 1.6%; n = 6), whereas the use-dependent block in 2 mM acetyl-KIFMK-amide was 62.4 ± 3.1% (n = 5).
We subsequently tested this peptide dissociation phenotype using the two-pulse protocol shown in Fig. 8 A with a 10-ms interval. The peak current amplitude at the second pulse was nearly the same as that at the first pulse (Fig. 8 A; dashed line). Evidently, the unblocking of β4154–167 occurred rapidly at −140 mV and probably reached its completion within 10 ms. Under identical conditions, the unblocking of acetyl-KIFMK-amide also occurred rapidly as shown in Fig. 8 B. Thus, for a short pulse of 3 ms, acetyl-KIFMK-amide elicited significantly less use-dependent block during repetitive pulses (28.5 ± 0.4%; n = 5 with the fitted τ = 14.5 pulses) than with a long pulse of 20 ms (Fig. 7 B; 62.4 ± 3.1%; P < 0.001). In fact, repetitive pulses at 50 mV for 1 ms at 1 Hz produced minimal use-dependent block (15.5 ± 0.4%; n = 5 with the fitted τ = 27.1 pulses). Evidently, the longer the pulse duration, the stronger the use-dependent block; this phenomenon suggests that the bound acetyl-KIFMK-amide could be moved to its second binding site during prolonged depolarization. In contrast, β4154–167 does not move further to such a second binding site under identical conditions. It is noteworthy that even with a short test pulse, the tail current induced by 2 mM β4154–167 decayed slower than that induced by 2 mM acetyl-KIFMK-amide (0.67 ± 0.04 ms vs. 0.23 ± 0.02 ms; n = 6). This phenotype implies that acetyl-KIFMK-amide dissociates faster than β4154–167 from the Na+ channel upon repolarization.
Figure 8.
Rapid unblocking of β4154–167 and acetyl-KIFMK-amide upon repolarization. (A) Unblocking of β4154–167 occurred within 10 ms as shown by a two-pulse protocol (top). The pipette solution contained 2 mM β4154–167. The interval was set for 10 ms between two pulses. The tail currents decayed rapidly to the baseline during this interval. Peak currents generated by these two pulses remained the same (dashed line). (B) Similar results were found in the presence of 2 mM acetyl-KIFMK-amide under identical conditions. Experiments in A and B were each repeated in six cells with the same results.
Competition between the β4 Peptide and the Inherent Fast Inactivation Particle
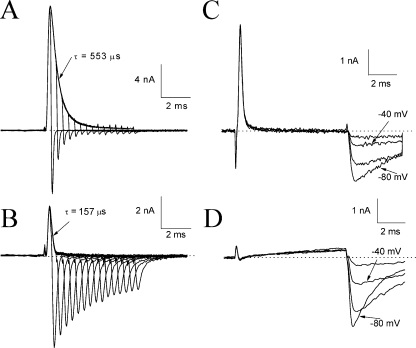
The intrinsic fast inactivation particle blocks the open Na+ channel rapidly (Aldrich et al., 1983). An elongated form of a large ShB peptide (i.e., the first 20 amino acids on the NH2 terminus of the Shaker channel) could enter deep into the inner cavity of the K+ channel as suggested by Zhou et al. (2001). According to the foot-in-the-door model for fast inactivation (Armstrong, 1981), the inactivated channel does not carry tail currents during deactivation because the activation gate (door) is partially closed before the withdrawal of the inactivation particle (foot). Fig. 9 A shows the representative traces of the wild-type hNav1.5 Na+ currents. The tail current amplitude is inversely proportional to the degree of fast inactivation during the pulse. The tail current through normal open Na+ channels decayed rather rapidly with a τ value of 94 ± 12 μs (n = 8 at pulse P1).
Figure 9.
Competition between β4154–167 and the intrinsic fast inactivation particle. Current traces of wild-type Na+ channels in the absence (A) and presence (B) of 1 mM β4154–167 were recorded at 50 mV with duration varying from 0.4 to 6.0 ms and were superimposed. The pulse protocol was the same as shown in Fig. 5 A. The outward current in A and B decayed with a τ value of 0.157 and 0.553 ms, respectively. Evidently, the time-dependent block induced by β4154–167 also occurred in wild-type Na+ channels. The tail current of trace 15 in A (P15) was barely detectable, but in B was quite large and significantly slower in its decay kinetics when 1 mM β4154–167 was present. Resurgent currents were recorded from wild-type Na+ channels at voltages ranging from −80 to −20 mV with a 20-mV step (C). The pulse protocol is the same as shown in Fig. 6 A. The pipette solution contained 1 mM β4154–167. Experiments were repeated in eight cells with similar results under the reversed Na+ gradient condition (65 mM Na+ ions in the bath and 130 mM Na+ ions in the pipette). (D) Current traces were recorded in the presence of 1 mM β4154–167 as described in C except that the bath and pipette solutions had 130 and 13 mM Na+ ions, respectively. Although Na+ currents at 50 mV were minimal under the normal Na+ gradient condition, the resurgent currents remained robust near the activation threshold. This experiment was repeated in five cells with similar results.
With 1 mM β4154–167 peptide in the pipette solution, this proportionality in the peak and tail currents was no longer true (Fig. 9 B). Also evident in this figure is that the decaying phase of the Na+ currents during the test pulse has a faster time constant (τ = 151 ± 15 μs; n = 8) than that without β4154–167 peptide (τ = 580 ± 13 μs; n = 6; P < 0.001; Fig. 9 A), suggesting that during depolarization, β4154–167 competes with the binding of the intrinsic inactivation particle. In addition, a peptide-induced tail current appeared upon repolarization. Near the activation threshold, resurgent Na+ currents appeared robust and long lasting (Fig. 9 C). Again, these resurgent tail currents could be attributed mostly to the rapid dissociation of the bound β4154–167 peptide. Such a tail current is absent from wild-type Na+ channels without the β4 subunit. These phenotypes are consistent with the hypothesis that the inactivation particle and the β4154–167 peptide are mutually exclusive in binding to their receptors (Raman and Bean, 2001).
Under a normal Na+ gradient (e.g., 130 mM of external Na+ ions and 13 mM of internal Na+ ions), competition between the intrinsic fast inactivation particle and β4154–167 peptide could not be observed readily during the 50-mV pulse (Fig. 9 D). The Na+ currents were small and difficult to identify under this condition because the pulse voltage was near the reversal potential (∼60 mV) and because a nonlinear leak was often present. However, resurgent tail Na+ currents were clearly evident near the activation threshold under the normal Na+ gradient and resembled those under the reversed gradient shown in Fig. 9 C, confirming again that competition occurs between β4154–167 peptide and the fast inactivation particle.
Changes in the Time-Dependent Block by External Na+ Ions
Afshari et al. (2004) reported that in cerebellar neurons, external Na+ ions could knock off the endogenous open-channel blocker, which induces resurgent currents. We tested whether a similar knock-off phenomenon can be detected in hNav1.5 Na+ channels. The time-dependent block of outward Na+ currents at 50 mV was measured under various external solutions containing 0, 65, and 130 mM Na+ ions. We found that the τ value for β4154–167 at 2 mM changed only slightly under these conditions (Fig. 10 A). The ratio of τ65/τ0 and τ130/τ0 was 1.09 ± 0.03 (n = 7) and 1.30 ± 0.04 (n = 7), respectively. These averaged values do deviate from unity significantly (P < 0.05). The peak currents were superimposed before and after the solution exchange. Tail currents were small or nonexistent in 0 mM Na+ ions, whereas tail currents were larger in external 130 mM than those in 65 mM Na+ ions. There were no significant changes in the tail current kinetics. Because the τ value for the time-dependent block by β4154–167 saturated at 2 mM and was severely limited by channel activation (Fig. 2 B), we also measured the Na+ effect at lower concentrations of β4154–167.
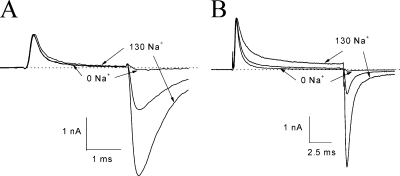
Figure 10.
Changes in the β4154–167 time-dependent block by external Na+ concentration. (A) Superimposed current traces of hNav1.5-CW mutant Na+ channels were recorded with a bath solution containing 130, 65, or 0 mM Na+ ions and normalized with respect to the outward peak value. The τ values for 0, 65, and 130 mM Na+ ions were 0.277 (τ0), 0.276 (τ65), and 0.334 ms (τ130), respectively. The pipette solution contained 2 mM β4154–167. A minimal tail current was present with 0 mM external Na+ ions, whereas a large tail was found with 65 and 130 mM external Na+ ions. (B) Currents were recorded under an identical protocol, except the pipette solution contained 125 μM β4154–167. The τ values for 0, 65, and 130 mM Na+ ions were 0.540, 0.697, and 0.952 ms, respectively. These experiments were repeated in five to seven cells.
Unlike at 2 mM, the τ values for β4154–167 at 125 μM were notably altered by external Na+ ions. Fig. 10 B shows that a decrease in external Na+ ions accelerates the time-dependent block, whereas an increase in external Na+ ions decelerates it. The ratio of τ65/τ0 and τ130/τ0 was 1.28 ± 0.05 (n = 5) and 1.81 ± 0.06 (n = 5), respectively. These ratios deviate from unity significantly (P < 0.05). In addition, the magnitude of steady-state block was higher at 0 mM Na+ ions and lower at 130 mM Na+ ions. These phenotypes were consistently found in cells with internal β4154–167 concentrations ranging from 25 to 250 μM. Evidently, external Na+ ions reduce internal β4154–167 binding strongly during depolarization. Interesting, unblocking of β4154–167 upon repolarization was not affected, as shown by similar kinetics of the tail currents (Fig. 10 B). This result implies that an increase in the Na+ concentration slows primarily the on rate binding of β4154–167. However, cautions must be taken in interpreting these results because the effect of external Na+ ions may be partially concealed at high β4154–167 concentrations, probably as a result of the saturated τ values shown in Fig. 2 B.
Minimal Time-Dependent Block of BTX-Modified Na+ Currents by β4154–167
BTX is a potent Na+ channel activator that eliminates the fast inactivation of voltage-gated Na+ channels (Catterall, 1980; Wang and Wang, 2003; for review see Strichartz, et al., 1987). To determine whether BTX also eliminates the time-dependent block induced by peptide blockers, we included 4 μM BTX in the pipette solution along with 2 mM acetyl-KIFMK-amide or 1 mM β4 peptide. With infrequent stimuli applied every 30 s, peptide-induced phenotypes, such as the time-dependent block during the test pulse and the large tail currents upon repolarization, were similar to those found without BTX. With repetitive pulses of 50 mV for 20 ms at 2 Hz, however, BTX began to bind to the open Na+ channel, and maintained BTX-modified Na+ currents became evident. Fig. 11(A and B) shows the current family at various voltages in wild-type BTX-modified Na+ channels after 2,000 repetitive pulses. Fast inactivation was severely impaired, and large Na+ currents were activated even at −80 mV. However, unlike traces shown in Figs. 7 and 8, little time-dependent block was found in these current traces despite the presence of 2 mM acetyl-KIFMK-amide and 1 mM β4154–167. The ratios of the maintained versus peak current at 50 mV were measured at 90.2 ± 3.9% (n = 6) and 89.2 ± 4.3% (n = 5) for experiments shown in Fig. 11(A and B, respectively). Similar results were found in BTX-modified inactivation-deficient mutant Na+ channels during a prolonged depolarization (Fig. 11 C); these currents again showed minimal time-dependent block by 1 mM β4154–167. In contrast, the time-dependent block by β4154–167 peptide was present before repetitive pulses. Evidently, the BTX-modified open Na+ channel becomes resistant to β4154–167 peptide and to acetyl-KIFMK-amide block. This result suggests that BTX prevents the intrinsic fast inactivation particle acetyl-KIFMK-amide and β4154–167 from binding with their receptors.
Figure 11.
Absence of time-dependent block by β4154–167 in BTX-modified wild-type or hNav1.5-CW mutant Na+ channels. Repetitive pulses (50 mV/20 ms at 2 Hz) were applied 2,000 times after the pipette break-in. The families of BTX-modified wild-type Na+ channels (A and B) or hNav1.5-CW mutant channels (C) were then recorded and superimposed. Current traces corresponding to 50 mV were labeled by arrows. Besides 4 μM BTX, the pipette solution also contained either 2 mM acetyl-KIFMK-amide (A) or 1 mM β4154–167 (B and C). The time frame was recorded ∼50× slower in C than that in A and B. The bath solution contained 130 mM Na+ ions (A) or 65 mM Na+ ions (B and C). Holding potential was set at −140 mV. Experiments were repeated in five to six cells with similar results.
Are hNav1.5-CW/F1760K Mutant Na+ Channels Resistant to β4154–167 Block?
The peptide-resistant phenotype in BTX-modified Na+ channels implies that the BTX receptor may overlap the β4154–167 receptor. This, in turn, raises a possibility that mutations at the BTX-binding site may also affect β4154–167 peptide binding. Therefore, we expressed BTX-resistant hNav1.5-CW/F1760K channels in HEK293t cells and examined their sensitivity toward β4154–167 peptide. The conserved F residue in hNav1.5-F1760 has been known to form a part of the local anesthetic receptor as well as the BTX receptor within the inner cavity of the Na+ channel (Ragsdale et al., 1994; Linford et al., 1998; Wang and Wang, 2003). Acetyl-KIFMK-amide may also interact with this aromatic residue (Wang and Wang, 2005). The location of this F residue is at the middle of D4S6, adjacent to the putative gating hinge (a serine residue; i.e., hNav1.5-S1759).
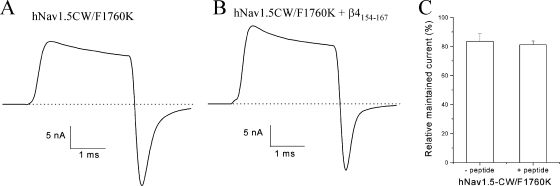
Fig. 12(A and B) shows traces of hNav1.5-CW/F1760K currents in the absence and presence of 1 mM β4154–167 peptide, respectively. The inactivation-deficient phenotype of hNav1.5-CW/F1760K channels is comparable with that of hNav1.5-CW counterparts. Unlike in hNav1.5CW channels, however, we did not observe a rapid time-dependent block by β4154–167 peptide in hNav1.5-CW/F1760K channels after internal dialysis for >30 min (Fig. 12 B). The relative maintained currents at the end of the test pulse in these mutant channels with the peptide remain the same as those without the peptide (Fig. 12 C; P = 0.68). In addition, we observed no significant changes in tail current kinetics during dialysis. Preliminary scanning of rNav1.4-WCW/F1579K Na+ currents at 50 mV also showed that these F1579K mutant Na+ channels were resistant to β4154–167 peptide at 1 mM. Our data thus reveal a locus critical for β4154–167 binding. Substitution of phenylalanine (F) with lysine (K) at this conserved D4S6 site appears to render both hNav1.5 and rNav1.4 isoforms not only resistant to LAs and BTX but also to β4154–167 peptide.
Figure 12.
A β4154–167-resistant phenotype in hNav1.5-CW/F1760K mutant Na+ channels. Traces of hNav1.5-CW/F1760K mutant Na+ currents at 50 mV were recorded after >30 min under whole-cell configuration in the absence (A) or presence (B) of 1 mM β4154–167 within the pipette solution. The τ values for the tail current decay were measured at 190.6 ± 30.0 μs and 219.7 ± 19.6 μs (n = 5) without and with 1 mM β4154–167, respectively. The difference in τ values is not statistically significant (P = 0.44). The maintained currents at the end of the test pulse in A and B were measured, normalized with the peak current amplitude, and plotted in C (n = 5). No statistical difference was found in these measurements. Error bars represent SD.
DISCUSSION
We have described several β4154–167-induced phenotypes in wild-type and inactivation-deficient mutant hNav1.5 Na+ channels. Many of these β4154–167-induced phenotypes resemble those found in native neuronal Na+ channels from Purkinje neurons (Grieco et al. 2005). First, cardiac Na+ channels can carry resurgent Na+ currents in the presence of intracellular β4154–167. Second, β4154–167 elicits a conspicuous time-dependent block at 50 mV. Third, β4154–167 dissociates rapidly from its receptor upon repolarization. Fourth, a fast inactivation peptide, acetyl-KIFMK-amide, induces resurgent Na+ currents almost as robustly as β4154–167. Fifth, BTX eliminates the time-dependent block elicited by β4154–167. Finally, lysine substitution of a conserved phenylalanine residue at D4S6 renders the channel resistant to β4154–167. We conclude that the hNav1.5 Na+ channel expressed in HEK293t cells is applicable to study the pharmacological actions of β4154–167. This in vitro expression system also provides the means for β4154–167 receptor mapping by site-directed mutagenesis. The origin of resurgent Na+ currents and the underlying mechanism of β4154–167 actions in Purkinje neurons have been documented in detail by Raman and Bean (2001) and Grieco et al. (2005). In the context of these landmark studies, the significance of β4154–167-induced phenotypes found in cardiac hNav1.5 channels is discussed in the following paragraphs.
Cardiac Nav1.5 Na+ Channels can Carry Resurgent Na+ Currents
Resurgent Na+ currents in dissociated cerebellar Purkinje neurons are probably carried by neuronal Nav1.6 Na+ channels (Raman, et al., 1997). Our results showed that cardiac and skeletal muscle inactivation-deficient mutant Na+ channels also carry robust resurgent Na+ currents in the presence of β4154–167. Evidently, resurgent currents can be generated in an in vitro expression system without dissociated neurons. It is likely that resurgent Na+ currents are not isoform specific, and most Na+ channel isoforms can carry resurgent Na+ currents. Transcripts of β4 have been found in the central nervous system, dorsal root ganglion, heart, and skeletal muscle (Yu et al., 2003). Immunostaining shows that β4 and Nav1.5 are colocalized at intercalated disks in ventricular myocytes (Maier et al., 2004). Whether the β4 subunit at intercalated disks can induce resurgent Na+ currents in vivo remains to be examined. Such a question has not yet been resolved in dissociated Purkinje neurons (Grieco et al. 2005). Coexpression of hNav1.5 and the β4 subunit in HEK293t cells may also help address this question in the future, although a positive result from this approach is by no means guaranteed because the native system could still turn out to require scaffolding proteins or some other accessory proteins to function properly (Bean, 2005).
β4154–167 Gains Access to its Receptor Only When the hNav1.5 Channel is Open
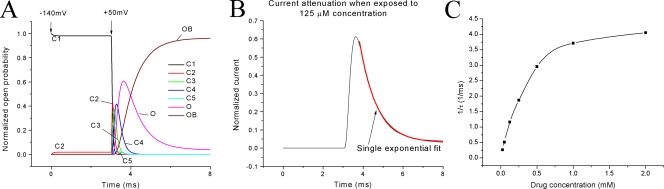
Several pieces of evidence support the hypothesis that the receptor for β4154–167 is inside the pore region of the hNav1.5 channel and that the activation gate governs the receptor accessibility. First, the time-dependent block of inactivation-deficient hNav1.5 Na+ currents at 50 mV (Fig. 1) suggests that β4154–167 interacts with the open Na+ channel directly as an open-channel blocker (Grieco et al. 2005). Second, external Na+ ions influence the kinetics of the time-dependent block by internal β4154–167 (Fig. 10 B). Such a phenomenon is often found for open-channel blockers and implies that a knock-off mechanism by external Na+ ions occurs in peptide binding within the pore region (Tang et al. 1996; Afshari et al. 2004). Third, when β4154–167 concentrations are ≥0.5 mM, the τ value of the time-dependent block becomes saturated (Fig. 2 B). Computer simulation shows that the block by β4154–167 at 125 μM is well fitted by a single exponential function (Fig.13 B). A nonlinear relationship with a saturation of the 1/τ value at high β4154–167 concentrations (≥0.5 mM) is observed by simulation (Fig. 13 C). What is the cause for this saturation at high peptide concentrations? We find that as the peptide concentration is raised, the τ value for the time-dependent block becomes faster and increasingly overlaps with the activation time course (Fig. 13 A). In other words, Na+ channels that open late will effectively delay the peptide binding and give the appearance of saturation of the 1/τ value. The results of this mathematical simulation strongly advocate that only the open channel is accessible to the β4154–167 block.
Figure 13.
Computer simulation of the time-dependent block by β4154–167 at 50 mV. (A) Normalized open probability against time is shown. Normalized probabilities for transient closed and open blocked states are also included. Data were generated by the MATLAB software according to Scheme 1 with α = 1.6 eV/40, β = 0.3 e−V/40, γ = 50 eV/100, and δ = 0.6 e−V/25, where V is in millivolts. The β4154–167 concentration is set at 125 μM; rate constants are the same as described in Fig. 2 A. (B) The time-dependent block during the pulse can be fitted by a single exponential function, τ = 0.86 ms. (C) The plot of 1/τ against β4154–167 concentration shows a nonlinear relationship. The difference on the saturation level between Fig. 2 B and Fig.13 C is likely inherent because rate constants for binding were extracted by linear regression and because the voltage coefficients were obtained by repetitive approximation.
β4154–167 Dissociates Rapidly from its Binding Site in the hNav1.5 Channel upon Repolarization
The occurrence of resurgent tail Na+ currents depends on the rapid dissociation of β4154–167 from its receptor upon repolarization (Raman and Bean, 2001; Grieco et al., 2005). Resurgent tail Na+ currents are robust and long lasting at the activation threshold (approximately −70 mV) as expected (Fig. 6). Depolarization to −70 mV alone without a conditioning pulse does not elicit large resurgent Na+ currents (Fig. 3). Also, no resurgent Na+ currents are found in wild-type hNav1.5 Na+ channels upon repolarization (Fig. 9 A) because the intrinsic fast inactivation particle does not unbind fast enough (Armstrong, 1981).
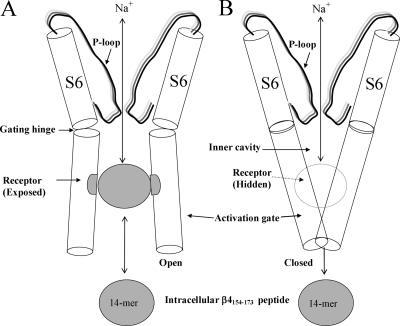
A fast inactivation peptide, acetyl-KIFMK-amide, can induce robust resurgent Na+ currents in inactivation-deficient hNav1.5 Na+ channels (Fig. 6 C), indicating that rapid dissociation of this small peptide causes resurgent currents to emerge upon repolarization. A small peptide such as acetyl-KIFMK-amide, however, may be trapped within the pore region by sequential reactions during repetitive pulses (Eaholtz et al., 1999), resulting in the additional use-dependent block of Na+ currents (Fig. 7, B and C). In contrast, a peptide such as β4154–167 could not be trapped within the inner cavity because of its large size. The rapid block and unblock of β4154–167 in hNav1.5 channels bears a clear resemblance to the block and unblock of polycyclic blockers in squid Na+ channels (Yeh and Narahashi, 1977; Cahalan and Almers, 1979). This resemblance can be adequately explained by the foot-in-the-door model (Fig. 14) proposed for the inherent fast inactivation particle (Armstrong, 1981).
Figure 14.
A cartoon for the foot-in-the-door model. The pore region of the pseudotetrameric α-subunit Na+ channel is formed by P-loop (S5/S6 linker) and S6 segments from each of four domains (D1–D4). The front and back structures are omitted for clarity. The inner cavity, the putative gating hinge, and the activation gate are labeled. The peptide receptor is exposed for binding (left) when the channel is in the open state (Tikhonov and Zhorov, 2005) and is hidden (right) when the channel is in the closed state (Lipkind and Fozzard, 2000). β4154–167 is drawn as a ball. The permeation pathway is not large enough to trap β4154–167 within the inner cavity when the channel is closed. Blocking and unblocking of β4154–167 within the pore is analogous to the foot-in-the-door model for fast inactivation proposed by Armstrong (1981). However, upon repolarization, the activation gate is partially deactivated before the withdrawal of the intrinsic fast inactivation particle from the channel mouth. In contrast, unbinding of β4154–167 is rapid upon repolarization, and during this dissociation process the activation gate remains open.
The Whereabouts of the β4154–167 Receptor in hNav1.5 Na+ Channels
The location of the β4154–167 receptor may be deduced by the properties of β4154–167 as an open-channel blocker, by competition studies with other ligands, and by site-directed mutagenesis. β4154–167 and acetyl-KIFMK-amide peptides act similarly as open-channel blockers in hNav1.5 channels. Both ligands produce strong time-dependent blocking of inactivation-deficient hNav1.5 channels at 50 mV (Fig. 8), and, upon repolarization, they both induce robust resurgent tail Na+ currents near the activation threshold (Fig. 6). The result that β4154–167 competes with the intrinsic fast inactivation particle for binding with the open pore (Fig. 9, A and B) is consistent with the hypothesis that occupancy of the hNav1.5 channel by β4154–167 prevents binding of the fast inactivation particle (Raman and Bean, 2001; Grieco et al., 2005), which contains the IFM motif.
Because BTX eliminates fast inactivation, we reason that BTX will also eliminate both acetyl-KIFMK-amide and β4154–167 binding within the inner cavity by physical exclusion. This prediction is supported by our data (Fig. 11); BTX-modified Na+ channels become resistant to blocking by both peptides. Because the BTX receptor is likely located within the inner cavity (Wang and Wang, 2003), Fig. 14 depicts the putative β4154–167 binding site, which is drawn between the gating hinge and the S6 COOH-terminal end. The COOH termini of S6s likely form the bundle-crossing site that acts as the activation gate (Fig. 14, right side; Lipkind and Fozzard, 2000; Jiang et al., 2002). The fact that a lysine substitution of a conserved phenylalanine residue (hNav1.5-F1760K) at the middle of the D4S6 segment (1) renders the channel resistant to β4154–167 (Fig. 12), (2) abolishes the use-dependent block by acetyl-KIFMK-amide, and (3) hinders its time-dependent block (Wang and Wang, 2005) are in agreement with this model.
Could this conserved F1760 residue form a part of receptors for β4154–167, acetyl-KIFMK-amide, local anesthetics, and BTX? This seems to be a logical explanation for the β4154–167-resistant phenotype in hNav1.5-F1760K mutant channels. Such an interpretation also agrees with most other β4154–167-induced phenotypes. Unfortunately, we were unable to rule out several other alternatives. First, a lysine substitution at F1760 could affect β4154–167 binding allosterically. Second, long-range charge–charge repulsion could occur between lysine (F1760K) and β4154–167, which has five net positive charges. Third, the β4154–167 receptor could overlap the BTX receptor only peripherally. These aforementioned scenarios do not require that F1760 interacts with β4154–167 directly and could place the β4154–167-binding site near the S6 COOH termini, closer to the intracellular entrance. Interestingly, a similar receptor site near the intracellular mouth has been suggested for the intrinsic fast inactivation particle in a hinged lid model (Catterall, 2000). Further detailed receptor mapping will be needed to delineate the receptor sites for β4154–167, acetyl-KIFMK-amide, and the fast inactivation particle with the IFM motif. All these open-channel blockers along with BTX appear to be mutually exclusive when bound within the pore region.
Acknowledgments
We thank Drs. Roland Kallen, William Catterall, and John Daly for providing us with hNav1.5, rβ1, and BTX, respectively.
This work was supported by grants from the National Institutes of Health (GM48090 and HL66076).
Olaf S. Andersen served as editor.
Abbreviations used in this paper: BTX, batrachotoxin; HEK, human embryonic kidney.
References
- Afshari, F.S., K. Ptak, Z.M. Khaliq, T.M. Grieco, N.T. Slater, D.R. McCrimmon, and I.M. Raman. 2004. Resurgent Na currents in four classes of neurons of the cerebellum. J. Neurophysiol. 92:2831–2843. [DOI] [PubMed] [Google Scholar]
- Aldrich, R.W., D.P. Corey, and C.F. Stevens. 1983. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 306:436–441. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M. 1981. Sodium channels and gating currents. Physiol. Rev. 61:644–683. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M., and F. Bezanilla. 1977. Inactivation of the sodium channel. II. Gating current experiments. J. Gen. Physiol. 70:567–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean, B.P. 2005. The molecular machinery of resurgent sodium current revealed. Neuron. 45:185–187. [DOI] [PubMed] [Google Scholar]
- Cahalan, M.D., and W. Almers. 1979. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychnine. Biophys. J. 27:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, S.C. 1996. Sodium channel defects in myotonia and periodic paralysis. Annu. Rev. Neurosci. 19:141–164. [DOI] [PubMed] [Google Scholar]
- Catterall, W.A. 1980. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 20:15–43. [DOI] [PubMed] [Google Scholar]
- Catterall, W.A. 2000. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 26:13–25. [DOI] [PubMed] [Google Scholar]
- Cota, G., and C.M. Armstrong. 1989. Sodium channel gating in clonal pituitary cells: the inactivation step is not voltage dependent. J. Gen. Physiol. 94:213–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins, T.R., S.D. Dib-Hajj, R.I. Herzog, and S.G. Waxman. 2005. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 579:2166–2170. [DOI] [PubMed] [Google Scholar]
- Do, M.T., and B.P. Bean. 2004. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J. Neurophysiol. 92:726–733. [DOI] [PubMed] [Google Scholar]
- Eaholtz, G., T. Scheuer, and W.A. Catterall. 1994. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron. 12:1041–1048. [DOI] [PubMed] [Google Scholar]
- Eaholtz, G., A. Colvin, D. Leonard, C. Taylor, and W.A. Catterall. 1999. Block of brain sodium channels by peptide mimetics of the isoleucine, phenylalanine, and methionine (IFM) motif from the inactivation gate. J. Gen. Physiol. 113:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco, T.M., and I.M. Raman. 2004. Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin. J. Neurosci. 24:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco, T.M., J.D. Malhotra, C. Chen, L.L. Isom, and I.M. Raman. 2005. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 45:233–244. [DOI] [PubMed] [Google Scholar]
- Hamill, O.P., E. Marty, M.E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Linford, N.J., A.R. Cantrell, Y. Qu, T. Scheuer, and W.A. Catterall. 1998. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA. 95:13947–13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind, G.M., and H.A. Fozzard. 2000. KcsA crystal structure as framework for a molecular model of the Na+ channel pore. Biochemistry. 39:8161–8170. [DOI] [PubMed] [Google Scholar]
- Maier, S.K., R.E. Westenbroek, K.A. McCormick, R. Curtis, T. Scheuer, and W.A. Catterall. 2004. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 109:1421–1427. [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado, R.D., and R.W. Aldrich. 1993. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J. Gen. Physiol. 102:949–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale, D.S., J.C. McPhee, T. Scheuer, and W.A. Catterall. 1994. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 265:1724–1728. [DOI] [PubMed] [Google Scholar]
- Raman, I.M., and B.P. Bean. 1997. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 17:4517–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, I.M., and B.P. Bean. 2001. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys. J. 80:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, I.M., L.K. Sprunger, M.H. Meisler, and B.P. Bean. 1997. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 19:881–891. [DOI] [PubMed] [Google Scholar]
- Strichartz, G., T. Rando, and G.K. Wang. 1987. An integrated view of the molecular toxinology of sodium channel gating in excitable cells. Annu. Rev. Neurosci. 10:237–267. [DOI] [PubMed] [Google Scholar]
- Tang, L., R.G. Kallen, and R. Horn. 1996. Role of an S4-S5 linker in sodium channel inactivation probed by mutagenesis and a peptide blocker. J. Gen. Physiol. 108:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov, D.B., and B.S. Zhorov. 2005. Sodium channel activators: model of binding inside the pore and a possible mechanism of action. FEBS Lett. 579:4207–4212. [DOI] [PubMed] [Google Scholar]
- Ukomadu, C., J. Zhou, F.J. Sigworth, and W.S. Agnew. 1992. μl Na+ channels expressed transiently in human embryonic kidney cells: biochemical and biophysical properties. Neuron. 8:663–676. [DOI] [PubMed] [Google Scholar]
- Wang, S.-Y., and G.K. Wang. 2003. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 15:151–159. [DOI] [PubMed] [Google Scholar]
- Wang, S.-Y., and G.K. Wang. 2005. Block of inactivation-deficient cardiac Na+ channels by acetyl-KIFMK-amide. Biochem. Biophys. Res. Commun. 329:780–788. [DOI] [PubMed] [Google Scholar]
- Wang, S.-Y., K. Bonner, C. Russell, and G.K. Wang. 2003. Tryptophan scanning of D1S6 and D4S6 C-termini in voltage-gated sodium channels. Biophys. J. 85:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J.W., D.E. Patton, T. Scheuer, Y. Wang, A.L. Goldin, and W.A. Catterall. 1992. A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc. Natl. Acad. Sci. USA. 89:10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, J.Z., and T. Narahashi. 1977. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J. Gen. Physiol. 69:293–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F.H., R.E. Westenbroek, I. Silos-Santiago, K.A. McCormick, D. Lawson, P. Ge, H. Ferriera, J. Lilly, P.S. DiStefano, W.A. Catterall, et al. 2003. Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. J. Neurosci. 23:7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M., J.H. Morals-Cabral, S. Mann, and R. MacKinnon. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661. [DOI] [PubMed] [Google Scholar]