Abstract
A key aspect of the lung's innate defense system is the ability of the superficial epithelium to regulate airway surface liquid (ASL) volume to maintain a 7-μm periciliary liquid layer (PCL), which is required for cilia to beat and produce mucus flow. The mechanisms whereby airway epithelia regulate ASL height to ≥7 μm are poorly understood. Using bumetanide as an inhibitor of Cl− secretion, and nystatin as an activator of Na+ absorption, we found that a coordinated “blending” of both Cl− secretion and Na+ absorption must occur to effect ASL volume homeostasis. We then investigated how ASL volume status is regulated by the underlying epithelia. Cilia were not critical to this process as (a) ASL volume was normal in cultures from patients with primary ciliary dyskinesia with immotile cilia, and (b) in normal cultures that had not yet undergone ciliogenesis. However, we found that maneuvers that mimic deposition of excess ASL onto the proximal airways, which occurs during mucociliary clearance and after glandular secretion, acutely stimulated Na+ absorption, suggesting that volume regulation was sensitive to changes in concentrations of soluble mediators in the ASL rather than alterations in ciliary beating. To investigate this hypothesis further, we added potential “soluble mediators” to the ASL. ASL volume regulation was sensitive to a channel-activating protein (CAP; trypsin) and a CAP inhibitor (aprotinin), which regulated Na+ absorption via changes in epithelial Na+ channel (ENaC) activity in both normal and cystic fibrosis cultures. ATP was also found to acutely regulate ASL volume by inducing secretion in normal and cystic fibrosis (CF) cultures, while its metabolite adenosine (ADO) evoked secretion in normal cultures but stimulated absorption in CF cultures. Interestingly, the amount of ASL/Cl− secretion elicited by ATP/ADO was influenced by the level of CAP-induced Na+ absorption, suggesting that there are important interactions between the soluble regulators which finely tune ASL volume.
INTRODUCTION
Mucus clearance is a major component of the lung's innate defense mechanism. The efficiency of mucus clearance reflects in part the volume of airway surface liquid (ASL) on airway surfaces. The ASL is comprised of a periciliary liquid layer (PCL), which lubricates the cell surface, and a mucus layer, which traps airborne particles and pathogens (Wine, 1999). Cystic fibrosis airways exhibit Na+ hyperabsorption and Cl− hyposecretion, which leads to ASL volume depletion, mucus stasis, and mucus plugging (Knowles and Boucher, 2002). These mucus plugs are the site of persistent bacterial infections that lead to a massive neutrophil influx and raised immune responses that promote airway remodeling (Chmiel and Davis, 2003). Regulation of ASL volume (height) is poorly understood (Tarran, 2004), reflecting the difficulties in measuring the physiology of this ∼7-μm layer. Although it is likely that ASL volume is controlled by active ion transport rather than “passive” physical forces (i.e., surface tension; Tarran et al., 2001a, 2005), it is not known how opposing Na+ absorptive and Cl− secretory ion transport pathways are coordinately regulated to mediated ASL volume homeostasis in normal (NL) or cystic fibrosis (CF) airways.
The superficial epithelia are usually described as predominantly Na+ and volume absorbing, largely based on electrophysiological studies in which the luminal surface is submerged in Ringer solution and native ASL is either diluted or washed away (Boucher, 1994). This dilution may be sufficient to remove the activity of molecules secreted into the ASL by the epithelia, which may regulate ion transport in an autocrine/paracrine fashion (Lazarowski et al., 2004; Tarran et al., 2005). For example, purine nucleotides and nucleosides regulate Na+ and Cl− transport via specific G protein–coupled receptors (Devor et al., 2000; Hentchel-Franks et al., 2004; Huang et al., 2001; Mason et al., 1991) and are present in the ASL at levels sufficient to activate the relevant receptors (Lazarowski et al., 2004). In addition, inhibition of apical membrane channel-activating proteins (CAPs), which regulate the activity of the epithelial Na+ channel (ENaC) by soluble inhibitors may also be an important component of the ASL volume homeostasis regulatory mechanism (Vallet et al., 1997; Bridges et al., 2001; Donaldson et al., 2002).
Superficial airway epithelia are not innervated by efferent neurons, implying that lungs are capable of local regulation of ASL volume (Alton et al., 1989). Wu et al. (1998) investigated this phenomenon in snap-frozen bovine tracheal strips by electron microscopy and found that ASL height approximated the height of outstretched cilia (∼6 μm). Addition of methacholine to this preparation caused a rapid, sevenfold increase in ASL height, due to glandular secretion, which was largely inhibited by blocking Cl− secretion with bumetanide. They also found that these secretions were spontaneously reabsorbed, presumably by the superficial epithelium and that this reabsorption was inhibited by amiloride pretreatment. These data suggested that ASL volume homeostasis occurs in epithelia isolated from the airways, and that active reabsorption of glandular secretions by the superficial epithelia is an important part of airway function. However, while glands are well known to secrete fluid, the mechanisms by which the superficial epithelia modify these secretions is not well understood.
To investigate mechanisms by which the superficial epithelia perform ASL volume homeostasis, we employed a well-differentiated bronchial culture system that possesses ciliated and goblet cells from the superficial epithelium and exhibits organized PCL and mucus layers (Matsui et al., 1998). We searched for potential regulatory mechanisms in these cultures by monitoring ASL height and the transepithelial potential difference (Vt), using confocal microscopy and microelectrodes, respectively. Coakley et al. (2003) have previously demonstrated that these primary cultures secrete insufficient bicarbonate (∼3–6 mM) to directly influence ASL height as compared with the ∼130 mM Cl− present in the ASL of these cultures (Tarran et al., 2001a,b). These low ASL bicarbonate levels may be a result of the low permeability for bicarbonate across the apical membrane (Willumsen and Boucher, 1992). Hence, after an initial investigation in bicarbonate-replete conditions, which confirmed these earlier data of Coakley et al. (2003), we performed most acute experiments under bicarbonate-free conditions.
Both NL and CF cultures were studied to assess the role of CF transmembrane conductance regulator (CFTR) in responding to volume regulatory signals. We first set out to test whether active ion transport does indeed set ASL volume (height) and to assess the relative contributions of Na+ absorption vs. Cl− secretion to ASL volume homeostasis. We then sought to determine how ASL volume is regulated by airway epithelia. Based on data from investigators of renal epithelia (e.g., Praetorius and Spring, 2003), we explored the possibility that cilia are capable of mechanically sensing ASL volume and signaling its status to the epithelia. In contrast, because we have previously shown that maintenance of ASL volume is strongly dependent on the presence of ASL nucleotides (ATP) and nucleosides (ADO) (Lazarowski et al., 2004; Tarran et al., 2005), we further explored the role that these and other soluble mediators of ion transport play in ASL volume regulation.
MATERIALS AND METHODS
Human Airway Epithelial Cultures
Cells were obtained from freshly excised bronchial specimens from NL and CF subjects by protease digestion (Matsui et al., 1998), seeded directly as primary cultures on 12-mm Transwell Col membranes (T-Col; Costar) in modified BEGM media under air–liquid interface conditions, and studied when fully differentiated (2–5 wk). For RT-PCR studies, cells were also grown on 24-mm T-col membranes. Cultures with transepithelial resistances (Rt) ≥300 Ωcm2 were studied and kept in a highly humidified incubator between readings (Matsui et al., 1998).
Solutions and Chemicals
During periods of XZ scanning and microelectrode recordings, cultures were bathed serosally in a modified Ringer solution (116 mM NaCl, 10 mM NaHCO3, 5.1 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 20 mM TES, 10 mM glucose, pH 7.4). At all other times during the experiments, cultures were maintained in a modified BEGM growth medium that contained 24 mM NaHCO and gassed with 5% CO2. PBS was used as an apical volume challenge and for washing the apical surface. No difference in ASL absorption rates were obtained over 48 h when either Ringer solution or PBS was used as the apical volume load (both n = 5). Amiloride (300 μM), anti-acetylated tubulin, aprotinin (1.5 units/ml), bumetanide (100 μM), nystatin (10 μM), trypsin (2 units/ml), and all salts were obtained from Sigma-Aldrich. All fluorescent compounds were obtained from Molecular Probes (USA) with the exception of the anti-mouse secondary antibody with was obtained from Jackson ImmunoResearch Laboratories. Dextran T-500 (500,000 D) was obtained from Amersham Biosciences.
Perfluorocarbon (PFC; FC-77) was obtained from 3M and had no effect on ASL height or Vt as previously reported (Tarran et al., 2001a,b). Bumetanide was dissolved as a concentrated stock in DMSO and diluted 1,000-fold in the serosal bath to give the final concentration (100 μM). ADO and ATP were added as dry powders suspended in PFC (FC-72) to give a final concentration of ∼300 μM (Tarran et al., 2001b).
Measurement of Transepithelial Potential Difference (Vt)
A macroelectrode (polyethylene tubing with 3 M KCl and 4% agar) was placed in the serosal bath and a 3 M KCl-filled glass microelectrode was positioned by a micromanipulator into the ASL to stably record Vt. Vts were recorded while the cultures were bathed in our low bicarbonate modified Ringer solution (see Solutions and Chemicals). PFC was added to the mucosal surface during the period of recording to avoid ASL evaporation (Tarran et al., 2001a).
Confocal Microscopy Measurement of ASL
To label the ASL, 20 μl PBS containing 2 mg/ml Texas red–dextran (10 kD) was added to the mucosal surface of the bronchial cultures. Note that cultures that spontaneously produced grossly visible mucus “hurricanes” and exhibited rotational mucus transport were excluded from this study to remove the confounding effects of the mucus reservoir effect (Tarran et al., 2001a). In some cases, i.e., before secretagogue addition, excess PBS was aspirated with a Pasteur pipette to acutely set ASL height at ∼7 μm. For all studies, 100 μl PFC was added mucosally to prevent evaporation of the ASL. Cultures were then placed in a chamber that had a serosal reservoir containing 80 μl of modified TES-buffered Ringer solution and placed on the stage of an inverted confocal microscope (either a Leica TCS 4D or Carl Zeiss MicroImaging, Inc. LSM 510) (Tarran et al., 2001a). This amount of PFC was selected since it was sufficient to cover the ASL during recordings yet evaporated soon after the cultures were returned to the highly humidified incubator. Cultures were imaged using a 63× water immersion lens. This approach yields a good working distance (220 μm) while maintaining a sufficiently high numerical aperture (1.2) to obtain high resolution XZ images suitable for resolving 3 vs. 7 μm ASLs. To measure the average height of the ASL, five predetermined points on the culture (one central, four circumferential) were XZ scanned. To increase the contrast between the ASL and the image background, the gain on the confocal microscope photomultiplier tube was adjusted to give an image intensity midway on an 8-bit image (i.e., ∼128 intensity units on a scale of 256 units). The offset was then reduced up to the point where the background was zero. This approach gave a high contrast image suitable for determination of ASL height.
Images were analyzed using either Metamorph (Universal Imaging) or ImageJ (NIH freeware) software by placing several regions of interest around the ASL that were then averaged for each image and the mean height calibrated based on a 5122 pixel image corresponding to 125 μm2. Regions of interest were consistently placed to avoid the 1-μm “fuzzy” interface between ASL image and background image. Preliminary investigations showed that this method of measuring ASL height yielded very reproducible results despite an intraculture variation in ASL height of up to 50%. For example, the mean change in ASL height was 8.8 ± 1.5 μm after 300 μM ATP addition to six cultures and 8.9 ± 1.4 μm when this maneuver was repeated on the same cultures ∼2 h later.
Immunohistochemical Methods
Airway cultures were fixed for 30 min at room temperature, in a solution of 4% (wt/vol) paraformaldehyde. After fixation, cultures were incubated in PBS containing 1% Triton X-100 for 20 min and washed in PBS. Cultures were then incubated with mouse anti-acetylated α tubulin for 15 min, washed in PBS, and incubated for 30 min with a secondary antibody (goat anti-mouse IgG-conjugated to Texas red). Specimens were then mounted in a chamber and imaged using a Carl Zeiss MicroImaging, Inc. 510 confocal microscope.
Real-time PCR Analysis
The T-col membrane containing the bronchial epithelium was removed from the surrounding support and placed in a microcentrifuge tube, and RNA was extracted using the standard protocol from the RNeasy mini kit (QIAGEN). Some cultures were stored in RNAlater (Ambion Inc.) at −20°C before extraction. The RNA was quantified by spectrometry, and a known quantity of RNA was used to make cDNA using SuperScript II (Invitrogen) and random primers. The cDNA was then diluted before real-time PCR, which was performed using the Roche LightCycler system and gene-specific primers. The reactions were incubated at 95°C for 10 min followed by 95°C for 0 s, 55°C for 5 s, and 72°C for 8 s for 45 cycles with a single fluorescence detection point at the end of the relevant annealing or extension segment. All reactions were run in duplicate. The absolute quantity of gene product/sample was obtained by comparing the crossing point (the point at which fluorescence crosses the detection threshold) of the gene products to the crossing points of known standards for each gene run at the same time as the samples. The standards were prepared by incorporating gene-specific PCR products into Topo-TA vectors. The copies/cell of each protease/protease inhibitor were calculated from the average crossing point of the sample and adjusting for RNA quantity and volume of the cDNA reaction.
Primer Sequences
The gene specific primer sequences were as follows (5′ to 3′): CAP1, forward, ctgtggccattctgctctatc, and reverse, acgccttcataggtgatgct; CAP2, forward, ctgaacagcctcgatgtcaa, and reverse, caagggacagtccagctctc; CAP3, forward, acgtcctgctcatcacactg, and reverse, ttctccccattgatctccac; HAT, forward, ttccagagctaaggcaagga, and reverse, cccagcttactatccccaca; HAI-1, forward, ggttcctggggttgtacctt, and reverse, gaagtgtaccgctcctaccg; and HAI-2, forward, cacgacttctgcctggtgt, and reverse, ggccactttcttgaggcact.
Statistics
Parametric statistics (ANOVA followed by the Tukey test to determine significant differences among groups) were used where the variances were homogeneously distributed. In the case of nonhomogeneity of variance, significance of difference between means was determined by either ANOVA followed by Dunn's Multiple Comparison Test, the Mann-Whitney U test, or the Wilcoxon Signed Rank Test as appropriate. All values are expressed as mean ± standard error where n represents the number of cultures (a minimum of three donors provided tissues per experiment). Based on a mean steady-state ASL height of 7.8 ± 0.4 (mean ± SEM; n = 9) for normal cultures, to detect a mean difference of 2.5 μm between groups (i.e., halfway between normal and CF), with an SD of 1.1 in each group (88% power, α 0.05, two-sided test), five cultures per group are required. Based on this calculation, we are powered to detect differences in ASL height of ∼3 μm based sample sizes of ∼n = 6.
RESULTS
ASL Volume Homeostasis Requires Active Na+ and Cl− Transport
Since airway epithelia possess regulatory components for both active Na+ absorption and Cl− secretion, we designed a series of experiments to test the hypothesis that regulation of both pathways is required to keep ASL volume at 7 μm in NL cultures. We have previously demonstrated that NL airway epithelia rapidly absorb excess Ringer solution and then maintain ASL volume at the height of extended cilia (∼7 μm; Tarran et al., 2001a, 2005). This volume homeostatic response reflects the adjustment of ion transport rates (i.e., a conversion of the epithelia from Na+ absorption to Cl− secretion), suggesting that airway epithelia are capable of homeostatic regulation of ASL volume (Tarran et al., 2001a, 2005). By comparison, CF airway epithelia exhibit uncontrolled absorption, resulting in a depleted ASL volume (Matsui et al., 1998; Tarran et al., 2005), suggesting that ion transport is unregulated in CF airway epithelia.
We tested for the contribution of Cl− secretion to ASL volume regulation by exposing cultures to bumetanide, an inhibitor of basolateral Cl− uptake (10−4 M) in the serosal bath for 48 h (Fig. 1, A and B). For these experiments, the cultures spent most of the 48-h exposure to bumetanide in a humidified incubator containing 5% CO2 and with 24 h mM NaHCO3 in the serosal bath. Cultures were taken out of the incubator for short periods (<10 min) and placed in a modified Ringer solution, containing TES as a buffer and 10 mM NaHCO3, to measure ASL height, and were promptly returned to the incubator and CO2/bicarbonate-buffered media.
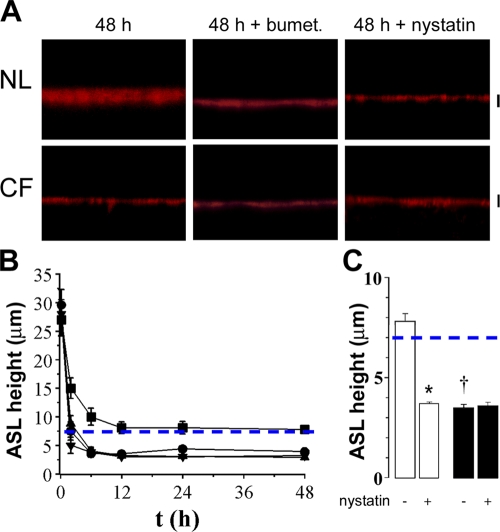
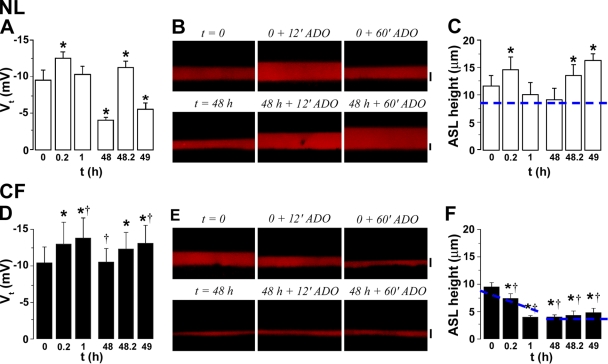
Figure 1.
Coordinated regulation of both Na+ and Cl− transport is required for NL ASL volume homeostasis. (A) XZ confocal images of ASL (red) 48 h after 20 μl PBS addition to NL and CF cultures, 48 h after bumetanide addition (100 μM, serosal) or 30 min after nystatin addition (10 μM, mucosal). (B) Mean data for NLs (squares; n = 6), NLs with bumetanide for 48 h (circles; n = 6), CF (upward triangles; n = 6), and CF with bumetanide cultures exposed to bumetanide for 48 h (downward triangles; n = 6). Note that NL ASL height was significantly higher (P < 0.05) than in the other three groups for all time points except t = 0; statistical symbols are not shown for clarity. (C) Mean data for NL (open bars; n = 7) and CF (closed bars; n = 8) cultures exposed to nystatin for 30 min 48 h after PBS addition. Dashed blue lines depict normal ASL height (i.e., 7 μm). Bars, 7 μm. Data shown as mean ± SEM. *, different (P < 0.05) from prenystatin values. †, CF different from NL (P < 0.05).
Under control conditions, NL cultures absorbed excess ASL within ∼12 h, after which time absorption ceased and ASL height was maintained at ∼7 μm (Fig. 1 A). In contrast, bumetanide addition both increased the initial rate of absorption (i.e., between 0 and 6 h) and reduced the steady-state ASL height from ∼7 to ∼3 μm (i.e., CF culture-like levels; Fig. 1, A and B). These data indicate that the ability to initiate Cl− secretion is vital for both controlling the rate of ASL absorption and maintaining the steady-state normal ASL height.
Consistent with Na+-dominated ASL hyperabsorption, CF cultures exhibited increased rates of absorption over time as compared with normal controls (Fig. 1, A and B). However, due to the absence of Cl− secretion in CF cultures under basal conditions (Tarran et al., 2005), bumetanide was without effect on CF cultures over the 48-h period.
Next, to test whether the inhibition of Na+ transport is required for ASL volume regulation, we exposed NL cultures to the ionophore nystatin (Garty, 1984) 48 h after 20 μl PBS addition, i.e., after steady state had been achieved, and measured ASL height 30 min later in cultures placed in a low bicarbonate modified Ringer solution (Fig. 1, A and C). In these experiments, normal airway epithelia absorbed excess liquid and maintained an ASL height of ∼8 μm for ∼48 h (Fig. 1, A and C). However, within 30 min of nystatin exposure, ASL height had decreased to 3.5 μm. These data suggest that the physiologic inhibition of active Na+ absorption seen under control conditions (Tarran et al., 2001a, 2005) was also critical for the normal adjustment of ASL height. In contrast, CF cultures exhibited unregulated Na+ absorption that depleted ASL volume to ∼3 μm within 6–12 h and nystatin was without further effect (Fig. 1, A and C).
Are Cilia Required for ASL Homeostasis in Bronchial Epithelial Cultures?
While these (Fig. 1) and previous data (Tarran et al., 2001a) strongly suggest that active ion transport is a critical determinant of ASL volume, it is not known if ASL volume status is signaled to the epithelia by a volume sensor. Because steady-state ASL and ciliary heights are similar, it is possible that cilia report ASL height by mechanically sensing changes in ASL volume and/or viscosity.
To test whether beating respiratory cilia were required to set ASL volume at 7 μm, we cultured bronchial epithelia from patients with immotile cilia syndrome (i.e., primary ciliary dyskinesia [PCD]), a genetic disorder that affects cilia, resulting in absent or uncoordinated beat patterns (Cole, 2001). After addition of a test solution (20 μl PBS), PCD cultures were able to regulate ASL volume to 7 μm, at rates indistinguishable from NL cultures (Fig. 2, A and B). Thus, beating cilia do not appear to be required to regulate ASL volume.
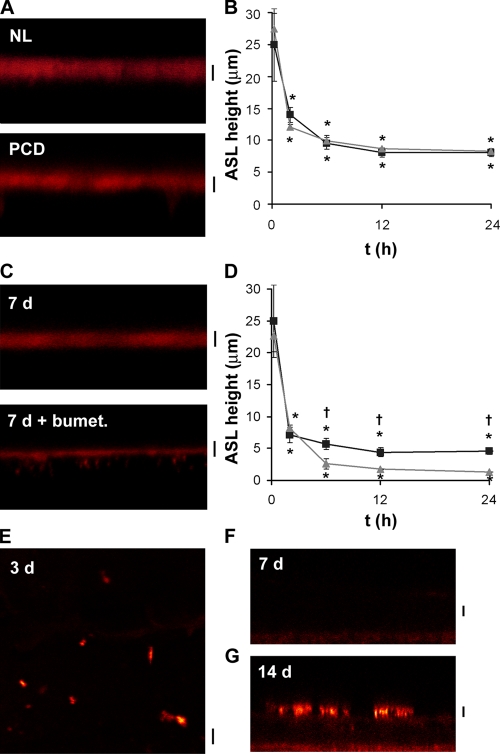
Figure 2.
Cilia are not required for ASL autoregulation. (A) XZ confocal images of ASL (red) 48 h after the addition of 20 μl PBS containing Texas red–dextran to ciliated NL and PCD cultures. (B) Mean ASL height with time after 20 μl PBS addition to NL (squares; n = 9) or PCD (circles; n = 7) cultures. (C) XZ confocal images of ASL 48 h after 20 μl PBS addition to preciliated (7 d old) NL cultures in the absence and presence of 10−4 M serosal bumetanide. (D) Mean ASL height with time after PBS addition. Preciliated cultures (upward triangles, n = 5) bumetanide-exposed cultures (downward triangles, n = 5). (E–G) Confocal micrographs showing anti-acetylated tubulin followed by Texas red secondary antibody staining in paraformaldehyde-fixed and Triton-X–permeabilized cultures. (E) XY image of 3-d-old NL airway culture. (F and G) XZ images of 7 and 14 d old cultures respectively. Note that tubulin could not be detected by either XZ or XY scans of 7-d-old NL bronchial cultures. Bars, 7 μm. *, different (P < 0.05) from t = 0. †, bumetanide-treated cultures different (P < 0.05) from control cultures.
Next, to determine whether cultures that completely lacked cilia were able to regulate ASL volume, we tested “young” cultures, which were confluent and exhibited active ion transport but had yet to become ciliated (∼5–9 d in culture; mean Rt was 443 ± 90 Ω.cm2; n = 5). These cultures also responded to 20 μl PBS addition by absorbing excess PBS, followed by a steady-state period of volume homeostasis (Fig. 2, C and D). To confirm that this steady-state height was maintained by active ion transport, a second set of nonciliated cultures were pretreated with 10−4 M serosal bumetanide, which resulted in depletion of virtually all the ASL (Fig. 2, C and D). Note that the steady-state ASL volume was lower in nonciliated cultures with bumetanide as compared with equivalent steady-state height in bumetanide-treated ciliated cultures. This slightly lower ASL volume in nonciliated cultures may reflect the absence of the space in the collapsed ASL compartment that is normally occupied by cilia.
Despite the preceding data, it is possible that nonciliated airway cultures still contain mechanosensitive primary cilia. Thus, to search for these structures, we probed cultures with an antibody against tubulin, to identify ciliary shafts. Staining with this antibody revealed the presence of primary cilia in both nonconfluent 2-d-old bronchial cultures (Fig. 2 E) and in A459 cells cultured on glass (not depicted). However, primary cilia were not detected in the confluent, nonciliated airway cultures used for the experiments shown in Fig. 2 (C and D) (Fig. 2 F). As expected, tubulin antibodies recognized cilia that had developed on well-differentiated (2 wk old) bronchial cultures (Fig. 2 G). Thus, 5–9-d-old cultures autoregulated ASL volume in the absence of either primary or motile cilia.
Is ASL Volume Homeostasis Dependent On Reporter Molecules Contained within the ASL?
Our hypothesis is that ATP, ADO, and channel-activating protein (CAP)/CAP inhibitors may act as signals that report changes in ASL volume. Their dilution, as occurs in Ussing chambers, would tend to inhibit Cl− secretion and activate Na+ absorption.
To test this hypothesis, we measured basal Vt under thin film conditions in cultures placed in a low bicarbonate modified Ringer solution (see Materials and Methods) (Tarran et al., 2001a), and then diluted ASL by adding 20 μl PBS, and remeasured Vt 1 h later to assess whether ion transport was altered. Indeed, 1 h after PBS addition, Vt had risen in NL cultures (Fig. 3 A). To differentiate between the possible effects of PBS addition on Vt induced by the dilution of soluble mediators in the ASL vs. a reduction in viscosity, which alters ciliary beating (Johnson et al., 1991), we also added 20 μl PBS with 15% T500 dextran (see Materials and Methods). This concentration of dextran has previously been shown to alter ciliary beat and activate TRPV4 Ca2+ channels (Johnson et al., 1991; Andrade et al., 2005). The T500 dextran/PBS effect on Vt over 1 h was not different from PBS alone (Fig. 3 A).
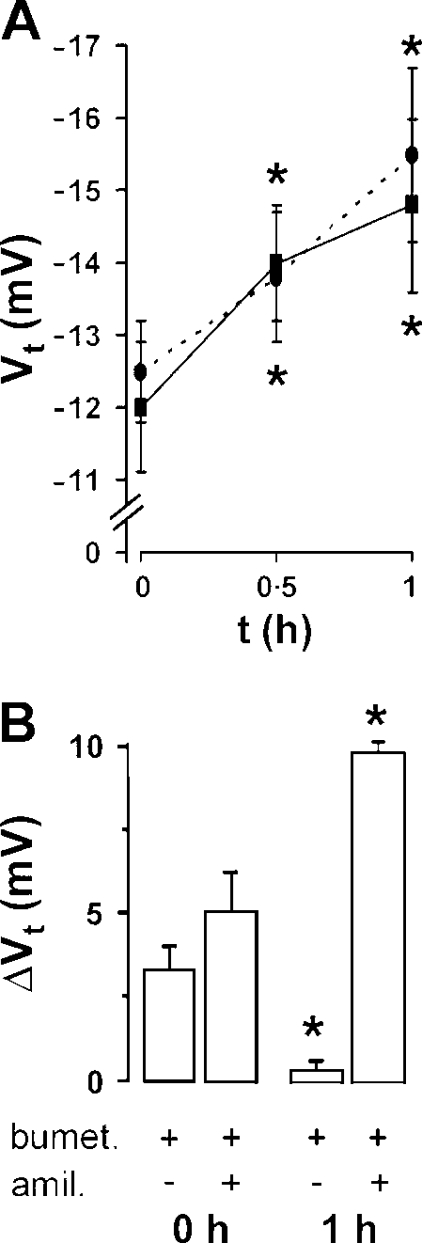
Figure 3.
ASL dilution stimulates Na+ absorption. (A) Transepithelial electric potential difference (Vt) across NL cultures with time. Solid line, before, and 1 h after apical addition of 20 μl PBS (n = 5). Broken line, before, and 1 h after apical addition of 20 μl PBS containing 15% T500 dextran (n = 6). (B) Changes in Vt in response to bumetanide (100 μM, serosal) and amiloride (300 μM, mucosal) in NL cultures (open bars, n = 8) before (0 h) and 1 h after PBS addition. *, different (P < 0.05) from t = 0.
To identify the relative contributions of changes in Cl− secretion vs. Na+ absorption to changes in Vt after PBS addition, we measured the amiloride-sensitive Vt (Na+ absorption) and bumetanide-sensitive Vt (Cl− secretion) during this sequence. The amiloride-sensitive Vt increased in NL cultures after 1 h and bumetanide-sensitive Vt (Cl− secretion) disappeared (Fig. 3 B), consistent with regulatory actions on both Cl− secretion (removal of activation) and Na+ absorption (activation).
Extracellular Protease Regulation of Na+ Transport as a Function of ASL Volume
We first searched for a role for CAPs and CAP inhibitors in regulating ion transport in human bronchial epithelial cultures under thin film conditions. Since we could not alter the expression levels of endogenous apical membrane CAPs in primary cultures, we probed this system with exogenous trypsin, a serine protease with known efficacy for ENaC to mimic CAP activity, and aprotinin, an inhibitor of CAP-mediated ENaC activation to mimic endogenous CAP inhibitors (Bridges et al., 2001; Donaldson et al., 2002).
Immediately after addition of “excess” ASL (20 μl PBS; t = 0) to normal cultures placed in a low bicarbonate modified Ringer solution, there was a relatively high basal Vt and a small (∼20%) increase with trypsin (Fig. 4 A). However, 48 h after 20 μl PBS addition, during which time, the cultures were kept in a bicarbonate-replete environment, basal Vt was reduced, whereas the trypsin-induced increase in Vt was significantly increased. These data suggest that slowing of Na+ transport-dependent ASL volume absorption may be associated with inhibition of CAP activity.
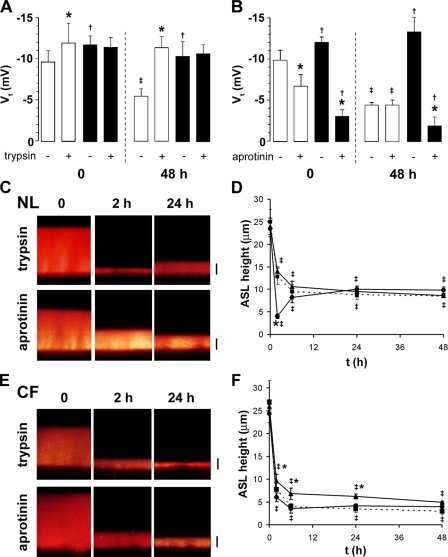
Figure 4.
Regulation of Na+ and ASL absorption by protease regulation. (A) Transepithelial electric potential difference (Vt) across NL (open bars) and CF cultures (closed bars) before and 30 min after apical trypsin (1.5 U/ml) addition at 0 or 48 h after PBS (20 μl) addition (n = 10 and 6 for NL, respectively; and 11 and 8 for CF, respectively). (B) Vt before and 30 min after apical aprotinin (2 U/ml) addition to NL (open bars) or CF (closed bars) cultures at t = 0 or 48 h after PBS addition (n = 5 and 5 for NLs; 4 and 4 for CFs, respectively). Note that all significant changes in Vt were abolished by amiloride pretreatment (3 × 10−4 M; all n = 4). (C) XZ confocal images of NL ASL (red) 0, 2, and 24 h after addition of 20 μl PBS containing Texas red–dextran with either 1.5 U/ml trypsin or 2 U/ml aprotinin. (D) Mean data taken from C. Untreated cultures (broken lines/squares, n = 7), trypsin (circles, n = 5), aprotinin (triangles, n = 5). (E) XZ confocal images of CF ASL 0, 2, and 24 h after addition of 20 μl PBS with either 1.5 U/ml trypsin or 2 U/ml aprotinin. (F) Mean data taken from E1. Untreated cultures (broken lines/squares, n = 6), trypsin (circles, n = 6), aprotinin (triangles, n = 6). *, different (P < 0.05) from pretryspsin or preaprotinin value. †, different (P < 0.05) between NL and CF cultures. ‡, different from t = 0 (P < 0.05). Bars, 7 μm.
To test this notion further, we applied the protease inhibitor aprotinin to NL cultures over the same time periods (Fig. 4 B). At t = 0, aprotinin was effective at reducing Vt, whereas at t = 48 h, aprotinin was ineffective. These observations are consistent with the hypothesis that an aprotinin-like endogenous inhibitor was concentrated in ASL as a function of time and inhibited CAPs. The net effect to inhibit CAP activity as a function of ASL volume reduction may reflect the fact that the inhibitor concentration increased more rapidly than cell surface CAP activity.
CF cultures failed to exhibit any trypsin-sensitive Vt at t = 0, and in contrast to NL epithelia, there was no decrease in Vt nor induction of trypsin-inducible Vt after 48 h of ASL volume hyperabsorption (Fig. 4 A). This observation suggests that CAP may not be inhibitable in airway epithelium in the absence of CFTR or that there was no inhibitor collecting in CF ASL with time. To distinguish between these possibilities, we applied aprotinin to CF airway epithelia. Aprotinin significantly inhibited Vt at both t = 0 and t = 48 h (Fig. 4 B), suggesting that the failure to inhibit ENaC activity with ASL volume depletion in CF reflected the absence of inhibitory activity in the ASL.
To test whether CAP regulation of ENaC could directly alter ASL volume homeostasis, we added either trypsin or aprotinin in 20 μl PBS to cultures at t = 0. As per the bumetanide-exposure experiments (Fig. 1), cultures were kept in a bicarbonate-replete environment for the majority of the 48 h and ASL height was quickly measured by confocal microscopy in a low bicarbonate modified Ringer solution, after which the cultures were returned to the bicarbonate-replete environment (i.e., the incubator). While aprotinin had little effect on ASL absorption rates over 48 h in NL cultures, trypsin addition significantly increased the initial rate of ASL volume absorption (Fig. 4, C and D). The aprotinin data imply that NL bronchial epithelial cultures have sufficient endogenous antiprotease activity to regulate ENaC to a lower level of activity at low ASL volumes. Trypsin could override the endogenous antiprotease activity but the effect appeared to have waned by 6 h (Fig. 3 D). However, in an additional group of cultures, further trypsin addition (as a dry powder in FC-77) at 24 h (i.e., when ASL height was 7 μm) resulted in a second collapse of ASL volume within 1 h to ∼3 μm (n = 6), suggesting that the transient effect reflected the inactivation of trypsin rather than cellular or ASL compensatory mechanisms.
Consistent with ENaC being maximally active in CF cultures, trypsin addition had no effect on the already high rates of ASL absorption over 48 h (Fig. 4, E and F). Aprotinin addition significantly slowed ASL absorption over 24 h (Fig. 4, E and F), However, due to a lack of basal Cl− secretion, ASL height still fell to ∼3 μm by 48 h, albeit at a reduced rate (Fig. 4, E and F).
To investigate which of the candidate CAPs/CAP inhibitors are expressed in this culture system, we performed quantitative real-time PCR analysis using primers designed against several potential CAPs and CAP inhibitors (see Table I and Rossier, 2004). CAP2 (TMPRSS4) and CAP3 (Matriptase) were the most highly expressed CAP proteases in NL and CF tissues at the mRNA level, while CAP-1 (prostasin) and human airway trypsin-like protease (HAT) were expressed at lower levels (Table I). CAP-1 and CAP-2 were both significantly up-regulated in CF cultures as compared with NLs, which perhaps reflected the persistent protease activity with time in CF cultures (Fig. 4). The known CAP inhibitors HAI-1 and HAI-2 were also expressed in both NL and CF cultures, and despite the lack of spontaneous CAP-inhibitor activity in CF cultures, HAI-1 was also significantly up-regulated in CF cultures (Table I).
TABLE I.
Message for Serine Proteases and Kunitz-type Protease Inhibitors
| Gene | Normal bronchial | CF bronchial |
|---|---|---|
| copies/cell | copies/cell | |
| CAP-1 (Prostasin) | 27.8 ± 11.2 (4) | 130.4 ± 36.5* (3) |
| CAP-2 (TMPRSS4) | 145.3 ± 48.5 (4) | 539.0 ± 212.9 (3) |
| CAP-3 (Matriptase) | 243.3 ± 13.4 (3) | 222.1 (2) |
| HAT (human airway trypsin-like protease) |
32.1 ± 7.0 (4) | 7.2 ± 0.8* (3) |
| HAI-1 | 87.7 ± 39.8 (4) | 289.7 ± 47.0* (3) |
| HAI-2 | 252.1 ± 74.6 (4) | 354.4 ± 92.9 (3) |
Primary bronchial epithelial cells were assessed for the quantity of RNA per cell of serine proteases (top) and protease inhibitors (bottom). Cells were cultured under air–liquid interface conditions and studied when fully differentiated (3–4 wk). The absolute quantity of message (copies/cell) was obtained by quantitative PCR using gene-specific primers. (n) denotes number of donors. * denotes P < 0.05, different between NL and CF cultures.
The Effects of Adenosine on Active Ion Transport and ASL Regulation in CF vs. Normal Cultures
Under static culture conditions, ATP release rates are low (∼300 fM/cm2/min), and most ATP at the steady state is converted metabolically to ADO (Lazarowski et al., 2004). Previous studies with inhibitors of ADO receptors have suggested that ADO is an important regulator of ASL volume (Lazarowski et al., 2004; Tarran et al., 2005). We therefore tested the actions of ADO directly on electrogenic ion transport (Vt) and ASL volume in NL vs. CF cultures. Since endogenous ADO-dependent regulation of ASL height was entirely bumetanide sensitive, we performed these experiments in our modified Ringer solution under low bicarbonate conditions. For these studies, exogenous ADO was added to the mucosal compartment as a dry powder at a concentration (3 × 10−4 M) predicted to maximally stimulate A2B receptors (Lazarowski et al., 1992).
Cultures were prelabeled with 20 μl PBS containing Texas red–dextran at t = 0, and excess PBS was immediately aspirated to artificially set ASL volume at ∼7 μm to allow direct comparisons between the changes in ASL height induced by ADO at t = 0 (presumably endogenous mediator “free”) vs. t = 48 h (after steady-state accumulation of endogenous mediators to regulate ASL height to 7 μm). The addition of ADO to NL cultures at t = 0 led to a small, rapid (∼0.2 h) increase in Vt (Fig. 5 A). At 48 h, when inhibition of Na+ transport was maximal (Tarran et al., 2001a, 2005), the ADO-induced increase in Vt was of significantly greater magnitude and duration. These changes in Vt were linked in NL cultures to increased ASL volume (Fig. 5, B and C): ADO at t = 0 induced a small and short-lived increased in ASL volume; at 48 h, when Na+ transport was inhibited, a larger increase in ASL secretion was detected. Thus, ADO in NL airway cultures induced electrogenic Cl− and volume secretion.
Figure 5.
Contrasting effects of adenosine on ion transport and ASL volume in NL and CF cultures. (A and D) Transepithelial PD (Vt) in NL (open bars, n = 6) and CF (closed bars, n = 5) cultures, respectively, after exposure to 300 μM mucosal adenosine (ADO) added at t = 0 and 48 h after mucosal PBS addition. Note that changes in NL but not CF Vt were inhibited by 10−4 M serosal bumetanide addition (all n = 3). (B and E) XZ confocal images of ASL before (0), 12 min, and 60 min after mucosal ADO addition (300 μM) to NL and CF cultures, respectively, at t = 0 or 48 h after 20 μl PBS addition. (C and F) Mean ASL height in NL (open bars, n = 7) and CF (closed bars, n = 8) cultures. The change in ASL height over 1 h of imaging in nontreated cultures is shown as a dashed line. 1 h untreated values for NL cultures were 9.0 ± 2.5 μm and 8.4 ± 1.2 μm (P < 0.05) at t = 1 h and t = 49 h, respectively (both n = 5). 1 h untreated values for CF cultures were 5.3 ± 0.4 μm (P < 0.05) and 4.0 ± 0.3 μm at t = 1 h and t = 49 h, respectively (both n = 7). *, significantly different from preADO (0, 48 h) value. †, data significantly different between NL and CF cultures. Bars, 7 μm.
Adenosine addition produced a distinctly different pattern of responses in CF cultures. At t = 0, ADO induced a sustained increase in Vt; at 48 h, a similar sustained increase in Vt was elicited by ADO (Fig. 5 D). Importantly, the ADO-induced increase in Vt in CF cultures at t = 0 was associated with a more rapid decrease in ASL volume as compared with baseline levels (Fig. 5, D–F). At 48 h, when all available liquid had been removed (ASL height ∼3 μm), the effect of ADO on ASL volume was negligible (despite the increased electrogenic transport). These studies are consistent with data from freshly excised CF airway epithelia that demonstrated that an increase in cellular cAMP increased the rate of Na+ transport (Boucher et al., 1986) and suggest that when there is liquid available for absorption (i.e., at t = 0), the already abnormally high rate of CF Na+-dependent volume absorption can be further accelerated.
Interactions between ADO/ATP and CAP Signaling Systems
Since the effectiveness of ADO on NL ASL secretion under low-bicarbonate conditions appeared to vary with time (Fig. 5) and coincided with the endogenous increase in CAP inhibitor activity (i.e., a decrease in ENaC activity; Fig. 4), we directly tested whether the CAP system, via regulation of ENaC activity, could influence ADO-regulated, Cl−-dependent ASL secretion. Although ATP is not present in the ASL at sufficient levels to activate P2Y2 receptors under static conditions, it is accumulated sufficiently to activate P2Y2 receptors under phasic motion (shear stress) conditions (Tarran et al., 2005). Because ATP may inhibit ENaC activity by a mechanism distinct from CAP activation, i.e., via P2Y2 receptor-mediated depletion of PIP2 (Yue et al., 2002; Kunzelmann et al., 2005), and thereby hyperpolarize the apical membrane sufficiently to induce Cl− secretion, we also tested the effects of ATP on ASL volume regulation. To this end, we pretreated NL and CF cultures with either trypsin or aprotinin and then for comparison, added either ADO or ATP and measured ASL responses.
In NL cultures, pretreatment with trypsin completely abolished ADO-mediated ASL volume secretion (Fig. 6, A and B), consistent with the notion that ENaC must be inhibited in order to hyperpolarize the apical membrane and generate a sufficient electrical driving force for Cl− secretion. Aprotinin pretreatment followed by ADO addition resulted in sustained Cl− secretion over 1 h in NL cultures, suggesting that inactivation of ENaC is indeed required to initiate Cl− secretion (Fig. 6, A and B).
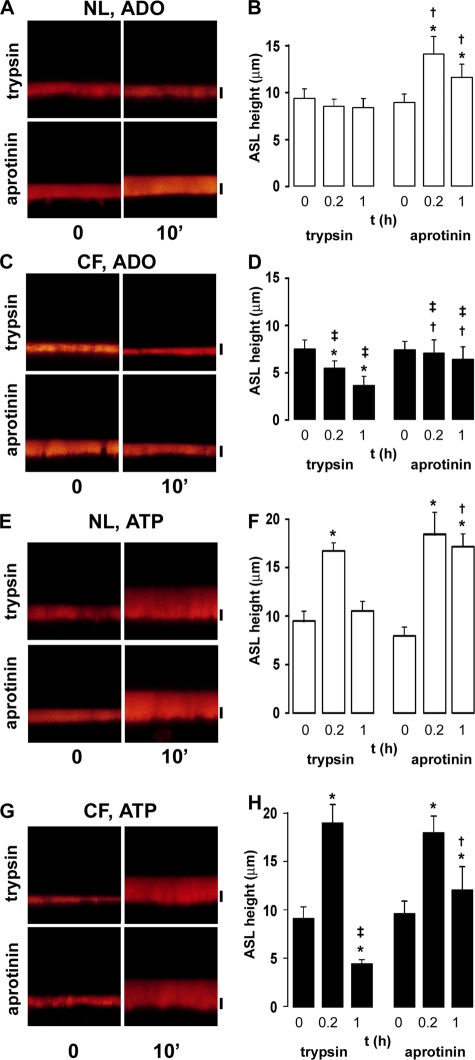
Figure 6.
Interaction between CAP and ADO/ATP signaling systems. (A) XZ confocal images of NL cultures acutely prewashed with PBS containing Texas red–dextran and either trypsin (1.5 U/ml) or aprotinin (2 U/ml) 0, 10, and 60 min post-ADO addition (300 μM). (B) Mean data taken from A. (C) XZ confocal images of CF cultures acutely prewashed with PBS/Texas red–dextran and either trypsin or aprotinin 0, 10, and 60 min after ADO addition. (D) Mean data taken from C. (E) XZ images of NL cultures prewashed with PBS/Texas red–dextran with either trypsin or aprotinin 0, 10, and 60 min after ATP addition (300 μM). (F) Mean data taken from E. (G) XZ confocal images of CF cultures prewashed with PBS/Texas red–dextran with either trypsin or aprotinin 0, 10, and 60 min after ATP addition. (H) Mean data taken from G. All data points are n = 6. *, different (P < 0.05) from t = 0. †, different (P < 0.05) from equivalent time point in the presence of tryspsin. ‡, different (P < 0.05) between NL and CF cultures. Bars, 7 μm.
In stark contrast, ADO addition resulted in volume hyperabsorption in CF cultures pretreated with trypsin (Fig. 6, C and D) as compared with NL controls (Fig. 6, A and B). This increase in absorption was similar to the effects of ADO without trypsin (Fig. 5, E and F). Aprotinin addition slowed ADO-induced absorption in CF cultures (i.e., prevented excessive volume absorption; Fig. 6, C and D). However, consistent with the absence of CFTR in CF tissues, ADO did not result in a net increase in ASL height (Fig. 6, C and D).
Important differences were seen in ATP-mediated ASL secretion in NL cultures with trypsin or aprotinin. Unlike with ADO, ATP was capable of evoking a volume response irrespective of whether ENaC was inhibited before ATP addition or not (Fig. 6, E and F). This initial ATP response was of equal magnitude after either trypsin or aprotinin pretreatment, suggesting that ATP can fully inhibit ENaC by an independent mechanism (i.e., depletion of intracellular PIP2; Yue et al., 2002). However, the duration of the ATP response differed; in the presence of trypsin, ATP caused a rapid increase in ASL height at 10 min that returned to baseline by 1 h, similar to the effects of UTP on NL airway epithelia (Tarran et al., 2001b). In the presence of aprotinin, ATP evoked an equal increase in ASL height, but the response was more sustained and ASL volume remained elevated for >1 h (Fig. 6, E and F), perhaps suggesting that the ATP had been metabolized to ADO by ecto-enzymes in the ASL (Picher et al., 2004).
ATP was also able to evoke an increase in ASL height in CF cultures in the presence of trypsin (Fig. 6, G and H). However, the response was short-lived and by 1 h, ASL height had fallen to ∼3 μm. Again, consistent with aprotinin inhibition of ENaC, the ATP response was longer lived in the presence of aprotinin (Fig. 6, G and H), consistent with the inhibitory actions of aprotinin on ENaC in CF cultures (Fig. 4).
DISCUSSION
Mechanical clearance of mucus is the dominant system that protects the airways from bacterial/viral infections, and the 7-μm-deep ASL is critical for promoting both ciliary-dependent and cough-dependent mucus clearance (Knowles and Boucher, 2002). A current hypothesis suggests that the pathogenesis of CF reflects depletion of ASL volume due to abnormal ion transport. However, the ability of the superficial epithelia to regulate ASL height by active ion transport is controversial (Verkman et al., 2003). Hence, the experimental strategy was designed to evaluate whether and how ASL volume was regulated by NL and CF surface airway epithelia after a modest ASL volume addition.
Regulation of ASL Volume by Active Ion Transport
The majority of previous studies on airway ion transport have been performed under “thick film” conditions in Ussing chambers where ASL has been diluted ∼105-fold by Ringer solution (Boucher, 1994). Two exceptions to this were studies by Wu et al. (1998) and Trout et al. (2003), who fixed bovine trachea and porcine bronchi, respectively, using techniques that preserved ASL morphology to allow scanning by EM. Wu et al. (1998) found that the regulation of ASL height was both amiloride and bumetanide sensitive in bovine trachea, and Trout et al. (2003) found that ASL height collapsed after combined bumetanide and dimethyl amiloride addition. Jayaraman et al. (2001) also investigated ASL height yet found no evidence for active ion transport regulating ASL height in tracheal epithelial cultures, although they did note that amiloride slowed ASL absorption. A possible explanation for these contradictory results is that Jayaraman et al. (2001) used preparations that retained large mucus layers on their surfaces. In our experience, the mucus gel can expand or contract depending on the volume of ASL present to maintain contact with cilia, making precise measurements of ASL volume difficult (Tarran et al., 2001a). Hence, our approach was to study ASL regulation in the absence of the mucus layer, reducing the noise in the measurement due to the characteristics of the mucus gel.
Using a preparation with a “thin film” of a predominantly isotonic salt solution, our data demonstrate that precise regulation of both Na+ absorption and Cl− secretion is required for normal ASL volume control (Fig. 1). Indeed, our data indicate that normal airway epithelia simultaneously generate (i.e., “blend”) active absorptive and secretory ion flows to finely control the quantity of salt and hence volume of liquid on airway surfaces. For example, the data that described an accelerated rate of initial volume absorption with bumetanide and a failure to regulate steady-state ASL height to 7 μm suggest that airways require continuous Cl− secretion to maintain ASL volume.
Willumsen and Boucher (1991) reported that addition of the nonselective ionophore amphotericin B to the apical membrane of airway cultures predominantly increases Na+ absorption (i.e., a mucosal to serosal Na+ flux) rather than increasing KCl secretion (serosal to mucosal Cl− or K+ flux). This preferential absorption of Na+ in airway epithelia likely occurs because the electrical gradient favoring Na+ entry is large, even after the addition of an ionophore, whereas the simultaneous electrochemical gradients for K+ and Cl− are relatively small. We predict that a similar result follows from nystatin addition, i.e., that an increased Na+ flux is the dominant effect. Consequently, to test whether bypassing the normal mechanism for the inhibition of Na+ absorption by nystatin addition would produce a failure of normal ASL volume regulation, we added nystatin to cultures at 48 h when ASL height was at steady state and ion transport rates (Vt) slowed. The capacity of nystatin to deplete ASL suggests that the ability to inhibit Na+ absorption with low ASL volumes is a key aspect of the overall ASL volume regulatory apparatus. Thus, our data strongly support the notion that active ion transport regulates the volume of liquid on normal airway surfaces.
In contrast, CF cultures exhibited an innate failure to regulate ASL volume (Fig. 1). This defect in regulation reflected abnormalities in both the Na+ absorption path (failure to inhibit absorption with low ASL volume) and the Cl− secretary path (failure to secrete Cl− due to an absence of CFTR). As would be predicted, CF epithelial ion transport and ASL volume regulation were unaffected by either bumetanide or nystatin. These data again confirm the importance of defective ion transport in failing to regulate CF ASL volume.
Cilia Are Not Required for ASL Height Regulation in Bronchial Airway Cultures
Investigators of renal epithelia have proposed that primary cilia act as mechanosensors (Praetorius and Spring, 2003; Yokoyama, 2004). While renal epithelia only express primary cilia, proximal airway epithelia express ∼200 motile cilia/ciliated cell so if primary cilia were also expressed, the ratio of motile to primary cilia would be 200:1. Thus, it is unlikely that primary airway cilia could detect volume-dependent vectoral flows upon the background of turbulent flows generated by beating, motile cilia. Indeed, we found no evidence that respiratory or primary cilia were required for ASL volume regulation using two different preparations. Cultures from patients with “immotile cilia syndrome” (PCD), and “young” airway cultures that had yet to undergo ciliogenesis yet were devoid of primary cilia, regulated ASL height to “normal” levels (Fig. 2). It remains to be determined whether sensors at the cell surface, such as microvilli, play a role as ASL volume sensors.
ASL volume was reduced by ∼2 μm in the absence of cilia (to ∼5 μm; Fig. 2). Based on their being 200 cilia per cell, each occupying a volume of 0.21 μm3, and their being 4 × 106 columnar cells per 1.1-cm2 culture, we calculated the volume occupied as the cilia as being 0.14 μl. We estimate that the volume of ASL at steady state is somewhere on the order of 1 μl. Thus, the absence of cilia would decrease ASL height/volume by 14%. That is, a 7.5-μm steady-state height would be reduced to 6.45 μm if the cilia were taken away. This is close to the observed 5-μm steady-state height observed in the absence of cilia (Fig. 2), suggesting that these slightly reduced ASL heights were due to the lack of volume normally occupied by cilia in the ASL. We have previously speculated that this volume occupied by cilia may be functionally important in passively retaining water on airway surfaces under conditions of extreme ASL depletion, as may occur in CF airways in the absence of effective phasic motion (Tarran et al., 2005).
Soluble Regulators of ASL Volume: CAPs (Proteases) and CAP Inhibitors
We identified several candidate CAPs and CAP inhibitors that are expressed in both NL and CF bronchial cultures using quantitative real-time PCR (Table I). While we cannot yet differentiate between them functionally, some of these proteins may be spontaneously active. For example, diluting the native ASL with 20 μl PBS increased the amiloride-sensitive Vt after 1 h (Fig. 3). This maneuver also decreased trypsin sensitivity in NL airways (Fig. 4 A). However, after 48 h, Vt declined (Fig. 4 A; Tarran et al., 2001a) and became much more sensitive to trypsin (Fig. 4 A). These data imply that (a) CAPs are tethered to the apical membrane, close to ENaC, and are not diluted after PBS addition, and that (b) CAP inhibitors are soluble and can be diluted after PBS addition, shifting the balance from secretion to absorption. Further, it is likely that soluble CAP inhibitors accumulated in the ASL with time under thin film conditions. Dilution of these endogenous CAP inhibitors likely explains why previous investigators failed to detect trypsin sensitivity in airway epithelia mounted in Ussing chambers (Bridges et al., 2001; Donaldson et al., 2002), as under these conditions, the volume of liquid bathing the apical surface (5 ml) likely diluted any naturally formed inhibitors (Bridges et al., 2001; Donaldson et al., 2002). In agreement with this interpretation, we detected aprotinin sensitivity of ENaC only after adding 20 μl PBS to airway surfaces, which presumably reflects the replacement of endogenous CAP inhibitors with an exogenous inhibitor (aprotinin). By comparison, when ENaC activity/Vt spontaneously declined with time, aprotinin sensitivity disappeared and the cultures became trypsin sensitive (Fig. 4).
CF airway cultures were trypsin insensitive over the entire 48-h period, suggesting that ENaC was maximally activated in these cultures and inappropriately so at 48 h (Fig. 4). The observation that Vt in CF cultures was inhibited by aprotinin suggests that ENaC responds normally to CAP inhibitors and implies that CF cultures exhibit a failure to inhibit CAPs over time, perhaps due to a failure to accumulate functional CAP inhibitors within the ASL.
We do not know which CAPs or their inhibitors are active in our culture system. However, Tong et al. (2004) searched for the identity of the protease(s) that regulate(s) ENaC activity in immortalized JME/CF15 cultures derived from CF ΔF508 nasal epithelia. These cultures lost protease regulation of ENaC after inhibition of prostasin (CAP-1) activity with siRNA. Prostasin is expressed equally in our NL and CF bronchial epithelial cultures. However, prostasin appears to be expressed at a much lower level in our cultures than TMPRSS4 (CAP2) and matriprase (CAP3). Unfortunately, primary epithelia are difficult to transfect, so a similar knockdown approach with siRNAs to test the relative importance of these CAPs in regulating ENaC is not feasible in our system.
We also detected two putative CAP inhibitors in our cultures (HAI-1 and HAI-2, also known as bikunin; Table I). These inhibitors were, if anything, expressed at higher mRNA levels, despite the lack of a functional inhibitor, in CF cultures. It is possible that the posttranslational modification/glycosylation of these inhibitors is altered in the absence of CFTR, rendering them inoperative, or that the disregulation of ENaC by ADO (cAMP) overrides any inhibition of CAPs (Fig. 4). While prostasin may be the predominant epithelial-derived protease (Tong et al., 2004), proteases may also be released from neutrophils, and it has recently been shown that neutrophil elastase is a potent activator of ENaC (Caldwell et al., 2005). Thus, infected CF airways may have multiple proteolytic pathways to persistently activate ENaC in the CF airway lumen.
Soluble Signals: ADO-dependent Regulation of ASL Volume
We have previously shown that inhibition of A2B adenosine receptors abolishes the ability of normal airway epithelia cultures to regulate ASL volume under standard static conditions (Lazarowski et al., 2004; Tarran et al., 2005). This finding is consistent with ADO being present in the ASL at levels suitable for stimulation of A2B receptors (100 nM, Tarran et al., 2005). In the airways, the A2B receptor has an EC50 of ∼1 μM (Huang et al., 2001), and we estimate that the NL ASL volume at steady-state height is ∼1 μl. Thus, a 20-fold dilution of the ASL after PBS addition would dilute ADO to ∼5 nM, which would be insufficient to stimulate A2B receptors and hence decrease Cl− secretion. In contrast, ENaC is active in NL airway epithelia under low cAMP conditions (Stutts et al., 1995) and would be predicted to be more active in the absence of ADO. Hence, assuming that ADO formation rates are constant, once ASL volume had been reduced via ENaC-mediated Na+ absorption, ASL ADO levels would return to the initial levels, reactivating Cl− secretion and reestablishing a steady-state ASL height as seen over 12–48 h post PBS addition (Fig. 1).
Addition of ADO to airway surfaces caused opposite ASL volume responses in NL and CF airway cultures. In normal airways, ADO raised Vt in a bumetanide-sensitive fashion and increased ASL height, consistent with ADO activation of CFTR (Fig. 5, A–C; Huang et al., 2001). The CF airway epithelium responded oppositely to nucleosides (ADO), i.e., ADO accelerated ASL absorption (Fig. 5, D–F). This response likely reflects the loss of CFTR's inhibitory effect on ENaC, which permitted direct cAMP activation of ENaC to dominate (Stutts et al., 1995). It is not yet known whether therapeutic agents that raise cell cAMP, e.g., β-agonists, will have similar potentially adverse effects on ASL volume regulation in CF patients.
Interactions between Soluble Signals: Nucleotides, Nucleosides, and CAPs/CAP Inhibitors
The ability of NL cultures under static culture conditions to secrete liquid and Cl− via ADO-mediated signaling increased as ENaC was endogenously inhibited with time (Fig. 5, A–C). Conversely, maximal activation of ENaC by trypsin addition abolished ADO-mediated ASL secretion (Fig. 6, A and B), mimicking the reduced effect of ADO at t = 0 (Fig. 5, A–C). Thus, while CFTR is usually thought of as an ENaC-mediated regulator of Na+ absorption rates (Stutts et al., 1995), under thin film/open circuit conditions, ENaC also appears to govern Cl− and ASL volume secretion. This relationship probably reflects the fact that under basal, i.e., t = 0 “high volume” conditions, there is no electrochemical driving force for Cl− secretion across the apical membrane of airway epithelia (Willumsen et al., 1989). The necessary driving force for Cl− secretion may be supplied with time when ENaC is endogenously inhibited by low ASL volume and the apical membrane is hyperpolarized by ENaC inhibition. Consequently, if ENaC is active, even maximal activation of CFTR Cl− conductance will fail to induce Cl− secretion.
We cannot fully exclude the possibility that these bronchial epithelial cultures would still secrete bicarbonate after ADO addition under conditions where Cl− secretion is inhibited (i.e., when ENaC is fully active). However, it appears that even under fully stimulated conditions (i.e., with forskolin and IBMX), these bronchial cultures do not secrete sufficient bicarbonate to significantly affect ASL height (ASL bicarbonate reached a maximum of 6.5 mM after 6 h of forskolin and IBMX exposure; Coakley et al., 2003). We will, however, add the caveat that the changes in pH from 6.2 to 6.8 (Coakley et al., 2003) may have subtle indirect effects on apical membrane proteins involved in ASL regulation that were not detected in our acute experiments performed under bicarbonate-free conditions.
Our findings with ATP differed. ATP appeared to be able to both inhibit ENaC and initiate Cl− secretion irrespective of the status of CAP/CAP inhibitor regulation of ENaC. These data suggest that relationships between ASL volume, ENaC regulation by CAPs/CAP inhibitors, and ATP will be more complex when studied under conditions of phasic motion, i.e., higher ATP release rate conditions (Tarran et al., 2005).
Conclusions
We have demonstrated that active Na+ absorption and Cl− secretion are required to regulate NL ASL volume, and inhibition of either pathway results in a failure to maintain ASL volume at heights sufficient to bathe cilia. Thus, our data point to the importance of active ion transport in regulating ASL volume, and suggest that NL superficial epithelia can adapt and rapidly absorb the excess ASL that accumulates on proximal surfaces by secretion from glands or after aerosol exposure.
In contrast, CF airway epithelia lack CFTR-dependent Cl− secretion and exhibit Na+ hyperabsorption, leaving CF cultures only partially able to adjust ASL volume (see Tarran et al., 2005). It is not clear whether the failure of CF cultures to regulate ASL height is reflected fully in a lack of an appropriate response by the effectors (ion channels) or in part also reflects a lack of a competent regulating system. ASL ATP and ADO levels appear to be normal in CF cultures, suggesting that the regulating system is functional (Lazarowski et al., 2004; Tarran et al., 2005). Clearly, the absence of one of the effectors (CFTR) limits the ability of CF airways to secrete ASL under low volume conditions and unfavorably alters the response of ENaC to ADO, further shifting the balance toward absorption. However, there is the perplexing question of why the CF CAP system appears unresponsive, and why CF cultures fail to induce trypsin sensitivity with time (Fig. 4 A), despite the apparent sensitivity of ENaC in CF cultures to exogenous CAP inhibitors (Fig. 4 B). Thus, in the case of the CAP/CAP inhibitor system, it would appear that the lack of CFTR attenuates a regulatory component of the system. It remains to be seen whether conditions that favor ASL volume regulatory systems that are still functional in CF patients, such as those that rely on ATP as the effector molecule (i.e., shear stress-dependent P2Y2 signaling; Tarran et al., 2005), will be able to sufficiently compensate for defective CAP signaling to regulate CF ASL volume.
Acknowledgments
Some of the data presented in this manuscript was obtained while R. Tarran was visiting the University of California at Berkeley and, accordingly, the support of Dr. T.E. Machen is gratefully acknowledged. We also thank the UNC CF Center Tissue Core for providing primary airway epithelial cells.
This work was supported by the Cystic Fibrosis Foundation and the National Institutes of Health (NIH) grants R01 HL074158 (R. Tarran) and K08 HL68617-03 (S.H. Donaldson), PPG HL34322-18 and SCOR P50 HL060280-08 (R.C. Boucher), and NIH R01DK51799 and CFF grant MACHEN03PO (T.E. Machen).
Lawrence G. Palmer served as editor.
Abbreviations used in this paper: ADO, adenosine; ASL, airway surface liquid; CAP, channel-activating protein; CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; ENaC, epithelial Na+ channel; NL, normal; PCD, primary ciliary dyskinesia; PCL, periciliary liquid layer; PFC, perfluorocarbon.
References
- Alton, E.W., A. Khagani, M.H. Yacoub, and D.M. Geddes. 1989. Lack of effect of lung denervation on the measurement of potential difference after single-lung transplantation. N. Engl. J. Med. 320:1755 (letter). [PubMed] [Google Scholar]
- Andrade, Y.N., J. Fernandes, E. Vazquez, J.M. Fernandez-Fernandez, M. Arniges, T.M. Sanchez, M. Villalon, and M.A. Valverde. 2005. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J. Cell Biol. 168:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, R.C. 1994. Human airway ion transport (Part 1). Am. J. Respir. Crit. Care Med. 150:271–281. [DOI] [PubMed] [Google Scholar]
- Boucher, R.C., M.J. Stutts, M.R. Knowles, L. Cantley, and J.T. Gatzy. 1986. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J. Clin. Invest. 78:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges, R.J., B.B. Newton, J.M. Pilewski, D.C. Devor, C.T. Poll, and R.L. Hall. 2001. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am. J. Physiol. 281:L16–L23. [DOI] [PubMed] [Google Scholar]
- Caldwell, R.A., R.C. Boucher, and M.J. Stutts. 2005. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am. J. Physiol. 288:L813–L819. [DOI] [PubMed] [Google Scholar]
- Chmiel, J.F., and P.B. Davis. 2003. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir. Res. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley, R.D., B.R. Grubb, A.M. Paradiso, J.T. Gatzy, L.G. Johnson, S.M. Kreda, W.K. O'Neal, and R.C. Boucher. 2003. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA. 100(26):16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, P. 2001. Pathophysiology and treatment of airway mucociliary clearance. A moving tale. Minerva Anestesiol. 67:206–209. [PubMed] [Google Scholar]
- Devor, D.C., R.J. Bridges, and J.M. Pilewski. 2000. Pharmacological modulation of ion transport across wild-type and deltaF508 CFTR-expressing human bronchial epithelia. Am. J. Physiol. 279:C461–C479. [DOI] [PubMed] [Google Scholar]
- Donaldson, S.H., A. Hirsh, D.C. Li, G. Holloway, J. Chao, R.C. Boucher, and S.E. Gabriel. 2002. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277:8338–8345. [DOI] [PubMed] [Google Scholar]
- Garty, H. 1984. Current-voltage relations of the basolateral membrane in tight amphibian epithelia: use of nystatin to depolarize the apical membrane. J. Membr. Biol. 77:213–222. [DOI] [PubMed] [Google Scholar]
- Hentchel-Franks, K., D. Lozano, V. Eubanks-Tarn, B. Cobb, L. Fan, R. Oster, E. Sorscher, and J.P. Clancy. 2004. Activation of airway Cl− secretion in human subjects by adenosine. Am. J. Respir. Cell Mol. Biol. 31:140–146. [DOI] [PubMed] [Google Scholar]
- Huang, P., E.R. Lazarowski, R. Tarran, S.L. Milgram, R.C. Boucher, and M.J. Stutts. 2001. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. USA. 98:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, S., Y. Song, L. Vetrivel, L. Shankar, and A.S. Verkman. 2001. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J. Clin. Invest. 107:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, N.T., M. Villalon, F.H. Royce, R. Hard, and P. Verdugo. 1991. Autoregulation of beat frequency in respiratory ciliated cells. Demonstration by viscous loading. Am. Rev. Respir. Dis. 144:1091–1094. [DOI] [PubMed] [Google Scholar]
- Knowles, M.R., and R.C. Boucher. 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways (“Perspective”). J. Clin. Invest. 109:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann, K., T. Bachhuber, R. Regeer, D. Markovich, J. Sun, and R. Schreiber. 2005. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 19:142–143. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., S.J. Mason, L.L. Clarke, T.K. Harden, and R.C. Boucher. 1992. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br. J. Pharmacol. 106:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski, E.R., R. Tarran, B.R. Grubb, C.A. van Heusden, S. Okada, and R.C. Boucher. 2004. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 279:36855–36864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, S.J., A.M. Paradiso, and R.C. Boucher. 1991. Regulation of transepithelial ion transport and intracellular calcium by extracellular adenosine triphosphate in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 103:1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, H., B.R. Grubb, R. Tarran, S.H. Randell, J.T. Gatzy, C.W. Davis, and R.C. Boucher. 1998. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 95:1005–1015. [DOI] [PubMed] [Google Scholar]
- Picher, M., L.H. Burch, and R.C. Boucher. 2004. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J. Biol. Chem. 279:20234–20241. [DOI] [PubMed] [Google Scholar]
- Praetorius, H.A., and K.R. Spring. 2003. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 12:517–520. [DOI] [PubMed] [Google Scholar]
- Rossier, B.C. 2004. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc. Am Thorac. Soc. 1:4–9. [DOI] [PubMed] [Google Scholar]
- Stutts, M.J., C.M. Canessa, J.C. Olsen, M. Hamrick, J.A. Cohn, B.C. Rossier, and R.C. Boucher. 1995. CFTR as a cAMP-dependent regulator of sodium channels. Science. 269:847–850. [DOI] [PubMed] [Google Scholar]
- Tarran, R. 2004. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc. Am. Thorac. Soc. 1:42–46. [DOI] [PubMed] [Google Scholar]
- Tarran, R., B. Button, M. Picher, A.M. Paradiso, C.M. Ribeiro, E.R. Lazarowski, L. Zhang, P.L. Collins, R.J. Pickles, J.J. Fredberg, and R.C. Boucher. 2005. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J. Biol. Chem. 280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran, R., B.R. Grubb, J.T. Gatzy, C.W. Davis, and R.C. Boucher. 2001. a. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J. Gen. Physiol. 118:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran, R., B.R. Grubb, D. Parsons, M. Picher, A.J. Hirsh, C.W. Davis, and R.C. Boucher. 2001. b. The CF salt controversy: In vivo observations and therapeutic approaches. Mol. Cell. 8:149–158. [DOI] [PubMed] [Google Scholar]
- Tong, Z., B. Illek, V.J. Bhagwandin, G.M. Verghese, and G.H. Caughey. 2004. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am. J. Physiol. 287:L928–L935. [DOI] [PubMed] [Google Scholar]
- Trout, L., M.I. Townsley, A.L. Bowden, and S.T. Ballard. 2003. Disruptive effects of anion secretion inhibitors on airway mucus morphology in isolated perfused pig lung. J. Physiol. 549:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet, V., A. Chraibi, H.P. Gaeggeler, J.D. Horisberger, and B.C. Rossier. 1997. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 389:607–610. [DOI] [PubMed] [Google Scholar]
- Verkman, A.S., Y. Song, and J.R. Thiagarajah. 2003. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. 284:C2–C15. [DOI] [PubMed] [Google Scholar]
- Willumsen, N.J., and R.C. Boucher. 1991. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am. J. Physiol. 261:C319–C331. [DOI] [PubMed] [Google Scholar]
- Willumsen, N.J., and R.C. Boucher. 1992. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J. Physiol. 455:247–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen, N.J., C.W. Davis, and R.C. Boucher. 1989. Intracellular Cl− activity and cellular Cl− pathways in cultured human airway epithelium. Am. J. Physiol. 256:C1033–C1044. [DOI] [PubMed] [Google Scholar]
- Wine, J.J. 1999. The genesis of cystic fibrosis lung disease. J. Clin. Invest. 103:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D.X.Y., C.Y.C. Lee, S.N. Uyekubo, H.K. Choi, S.J. Bastacky, and J.H. Widdicombe. 1998. Regulation of the depth of surface liquid in bovine trachea. Am. J. Physiol. 274:L388–L395. [DOI] [PubMed] [Google Scholar]
- Yokoyama, T. 2004. Motor or sensor: a new aspect of primary cilia function. Anat. Sci. Int. 79:47–54. [DOI] [PubMed] [Google Scholar]
- Yue, G., B. Malik, G. Yue, and D.C. Eaton. 2002. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J. Biol. Chem. 277:11965–11969. [DOI] [PubMed] [Google Scholar]