Abstract
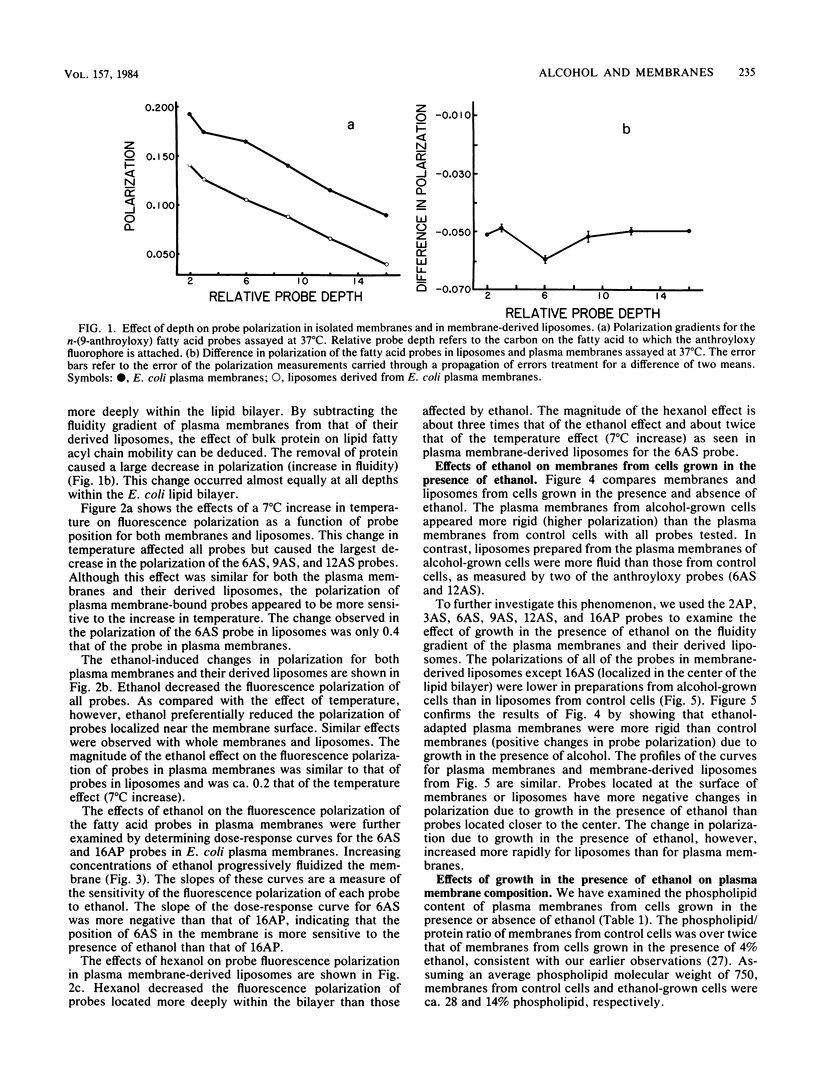
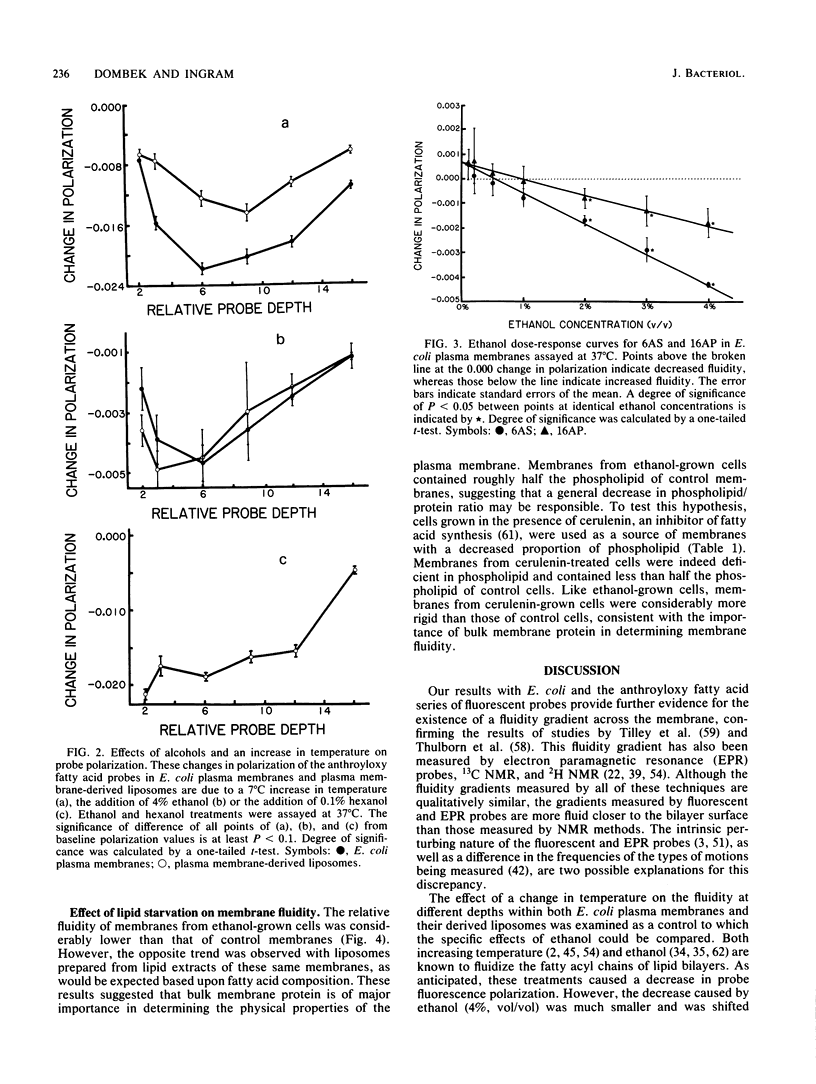
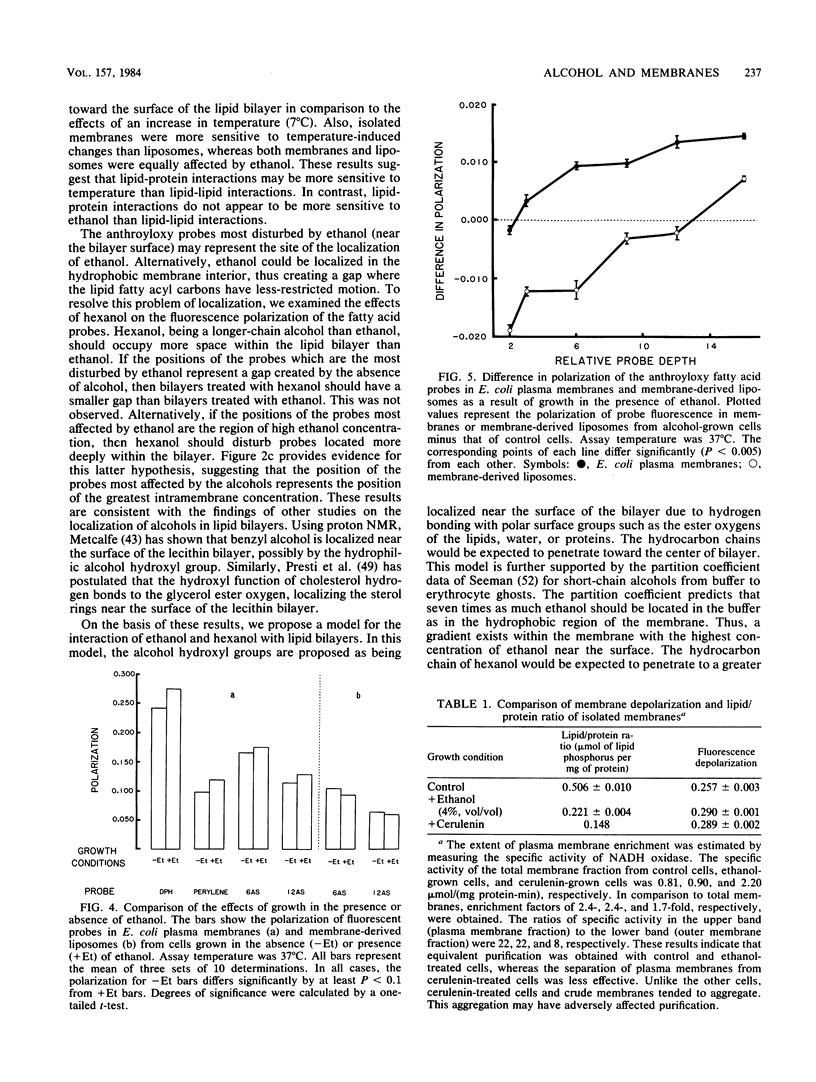
The effects of ethanol on the fluidity of Escherichia coli plasma membranes were examined by using a variety of fluorescent probes: 1,6-diphenyl-1,3,5-hexatriene, perylene, and a set of n-(9-anthroyloxy) fatty acids. The anthroyloxy fatty acid probes were used to examine the fluidity gradient across the width of the plasma membrane and artificial membranes prepared from lipid extracts of plasma membranes. Ethanol caused a small decrease in the polarization of probes primarily located near the membrane surface. In comparison, hexanol decreased the polarization of probes located more deeply in the membrane. Temperature had a large effect on probes located at all depths. The effects of ethanol on E. coli membranes from cells grown with or without ethanol were also examined. Plasma membranes isolated from cells grown in the presence of ethanol were more rigid than those from control cells. In contrast to plasma membranes, artificial membranes prepared from lipid extracts of ethanol-grown cells were more fluid than those from control cells. These differences are explained by analyses of membrane composition. Membranes from cells grown in the presence of ethanol are more rigid than those from control cells due to a decrease in the lipid-to-protein ratio. This change more than compensates for the fluidizing effect of ethanol and the ethanol-induced increase in membrane C18:1 fatty acid which occurs during growth. Our results suggest that the regulation of the lipid-to-protein ratio of the plasma membrane may be an important adaptive response of E. coli to growth in the presence of ethanol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzini A. F., Ingram L. O., Clem L. W. Temperature-mediated processes in teleost immunity: homeoviscous adaptation in teleost lymphocytes. Proc Soc Exp Biol Med. 1982 Jan;169(1):12–18. doi: 10.3181/00379727-169-41300. [DOI] [PubMed] [Google Scholar]
- Andrich M. P., Vanderkooi J. M. Temperature dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phophoslipid artificial membranes. Biochemistry. 1976 Mar 23;15(6):1257–1261. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Badley R. A., Martin W. G., Schneider H. Dynamic behavior of fluorescent probes in lipid bilayer model membranes. Biochemistry. 1973 Jan 16;12(2):268–275. doi: 10.1021/bi00726a015. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Morgan C. G., Radda G. K. Measurement and interpretation of fluorescence polarisations in phospholipid dispersions. Biochim Biophys Acta. 1976 Mar 5;426(2):157–172. doi: 10.1016/0005-2736(76)90329-1. [DOI] [PubMed] [Google Scholar]
- Berger B., Carty C. E., Ingram L. O. Alcohol-induced changes in the phospholipid molecular species of Escherichia coli. J Bacteriol. 1980 Jun;142(3):1040–1044. doi: 10.1128/jb.142.3.1040-1044.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vivo. Biochemistry. 1978 Feb 21;17(4):637–644. doi: 10.1021/bi00597a012. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Cadenhead D. A., Kellner B. M., Müller-Landau F. A comparison of a spin-label and a fluorescent cell membrane probe using pure and mixed monomolecular films. Biochim Biophys Acta. 1975 Mar 13;382(2):253–259. doi: 10.1016/0005-2736(75)90183-2. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Cameron D. G., Smith I. C., Mantsch H. H. Acholeplasma laidlawii membranes: a Fourier transform infrared study of the influence of protein on lipid organization and dynamics. Biochemistry. 1980 Feb 5;19(3):444–451. doi: 10.1021/bi00544a007. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Dodd G. H., Chapman D. Small molecule-lipid membrane interactions and the puncturing theory of olfaction. Biochim Biophys Acta. 1970 Sep 15;211(3):409–416. doi: 10.1016/0005-2736(70)90246-4. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Membrane-disordering action of ethanol: variation with membrane cholesterol content and depth of the spin label probe. Mol Pharmacol. 1981 May;19(3):425–431. [PubMed] [Google Scholar]
- Clark D. P., Beard J. P. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J Gen Microbiol. 1979 Aug;113(2):267–274. doi: 10.1099/00221287-113-2-267. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton L. C., Tedder T. F., Ingram L. O. Effects of fatty acid composition on the sensitivity of membrane functions to ethanol in Escherichia coli. Subst Alcohol Actions Misuse. 1982;3(1-2):77–87. [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Gilmore J. R., Glaser M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid compositions. Biochemistry. 1977 May 3;16(9):1881–1890. doi: 10.1021/bi00628a019. [DOI] [PubMed] [Google Scholar]
- Goldstein D. B., Chin J. H. Disordering effect of ethanol at different depths in the bilayer of mouse brain membranes. Alcohol Clin Exp Res. 1981 Spring;5(2):256–258. doi: 10.1111/j.1530-0277.1981.tb04898.x. [DOI] [PubMed] [Google Scholar]
- Herreman W., van Tornout P., van Cauwelaert F. H., Hanssens I. Interaction of alpha-lactalbumin with dimyristoyl phosphatidylcholine vesicles. II. A fluorescence polarization study. Biochim Biophys Acta. 1981 Jan 22;640(2):419–429. doi: 10.1016/0005-2736(81)90467-3. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Preferential inhibition of phosphatidyl ethanolamine synthesis in E. coli by alcohols. Can J Microbiol. 1977 Jun;23(6):779–789. doi: 10.1139/m77-115. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Regulation of fatty acid composition in Escherichia coli: a proposed common mechanism for changes induced by ethanol, chaotropic agents, and a reduction of growth temperature. J Bacteriol. 1982 Jan;149(1):166–172. doi: 10.1128/jb.149.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Vreeland N. S. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J Bacteriol. 1980 Nov;144(2):481–488. doi: 10.1128/jb.144.2.481-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Vreeland N. S., Eaton L. C. Alcohol tolerance in Escherichia coli. Pharmacol Biochem Behav. 1980;13 (Suppl 1):191–195. doi: 10.1016/s0091-3057(80)80030-x. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wobschall D. Rotation of fluorescent probes localized within lipid bilayer membranes. Chem Phys Lipids. 1974 Apr;12(2):117–131. doi: 10.1016/0009-3084(74)90049-8. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KATES M., KUSHNER D. J., JAMES A. T. The lipid composition of Bacillus cereus as influenced by the presence of alcohols in the culture medium. Can J Biochem Physiol. 1962 Jan;40:83–94. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lancée-Hermkens A. W., de Kruijff B. 13 C NMR measurements of unsonicated phosphatidylcholine bilayers of different fatty acid and sterol composition. Biochim Biophys Acta. 1977 Oct 17;470(2):141–151. doi: 10.1016/0005-2736(77)90095-5. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Warren G. B., Roberts G. C. A determination of the mobility gradient in lipid bilayers by 13C nuclear magnetic resonance. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):253–274. doi: 10.1098/rspb.1976.0045. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Polnaszek C. F. Spin-label and deuterium order parameter discrepancies in bilayers: one possible explanation. Biochemistry. 1978 May 2;17(9):1758–1760. doi: 10.1021/bi00602a028. [DOI] [PubMed] [Google Scholar]
- Nandini-Kishore S. G., Mattox S. M., Martin C. E., Thompson G. A., Jr Membrane changes during growth of Tetrahymena in the presence of ethanol. Biochim Biophys Acta. 1979 Mar 8;551(2):315–327. doi: 10.1016/0005-2736(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Nichol C. P., Davis J. H., Weeks G., Bloom M. Quantitative study of the fluidity of Escherichia coli membranes using deuterium magnetic resonance. Biochemistry. 1980 Feb 5;19(3):451–457. doi: 10.1021/bi00544a008. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pang K. Y., Chang T. L., Miller K. W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol. 1979 May;15(3):729–738. [PubMed] [Google Scholar]
- Podo F., Blasie J. K. Nuclear magnetic resonance studies of lecithin bimolecular leaflets with incorporated fluorescent probes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1032–1036. doi: 10.1073/pnas.74.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti F. T., Pace R. J., Chan S. I. Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry. 1982 Aug 3;21(16):3831–3835. doi: 10.1021/bi00259a017. [DOI] [PubMed] [Google Scholar]
- Rigomier D., Bohin J. P., Lubochinsky B. Effects of ethanol and methanol on lipid metabolism in Bacillus subtilis. J Gen Microbiol. 1980 Nov;121(1):139–149. doi: 10.1099/00221287-121-1-139. [DOI] [PubMed] [Google Scholar]
- Schreier S., Polnaszek C. F., Smith I. C. Spin labels in membranes. Problems in practice. Biochim Biophys Acta. 1978 Dec 15;515(4):395–436. doi: 10.1016/0304-4157(78)90011-4. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. C. The Ayerst Award Lecture 1978. Organization and dynamics of membrane lipids as determined by magnetic resonance spectroscopy. Can J Biochem. 1979 Jan;57(1):1–14. doi: 10.1139/o79-001. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Bister K., Schreiber C., Tunggal B. 13C-NMR studies of the membrane structure of enveloped virions (vesicular stomatitis virus). Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):905–915. doi: 10.1515/bchm2.1976.357.2.905. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Sawyer W. H. Properties and the locations of a set of fluorescent probes sensitive to the fluidity gradient of the lipid bilayer. Biochim Biophys Acta. 1978 Aug 4;511(2):125–140. doi: 10.1016/0005-2736(78)90308-5. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Tilley L. M., Sawyer W. H., Treloar F. E. The use of n-(9-anthroyloxy) fatty acids to determine fluidity and polarity gradients in phospholipid bilayers. Biochim Biophys Acta. 1979 Dec 4;558(2):166–178. doi: 10.1016/0005-2736(79)90057-9. [DOI] [PubMed] [Google Scholar]
- Tilley L., Thulborn K. R., Sawyer W. H. An assessment of the fluidity gradient of the lipid bilayer as determined by a set of n-(9-anthroyloxy) fatty acids (n = 2, 6, 9, 12, 16). J Biol Chem. 1979 Apr 25;254(8):2592–2594. [PubMed] [Google Scholar]
- Utsumi H., Tunggal B. D., Stoffel W. Carbon-13 nuclear magnetic resonance studies on the interaction of glycophorin with lecithin in reconstituted vesicles. Biochemistry. 1980 May 27;19(11):2385–2390. doi: 10.1021/bi00552a016. [DOI] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M. Effect of ethanol on membranes: a fluorescent probe study. Alcohol Clin Exp Res. 1979 Jan;3(1):60–63. doi: 10.1111/j.1530-0277.1979.tb04770.x. [DOI] [PubMed] [Google Scholar]


