Abstract
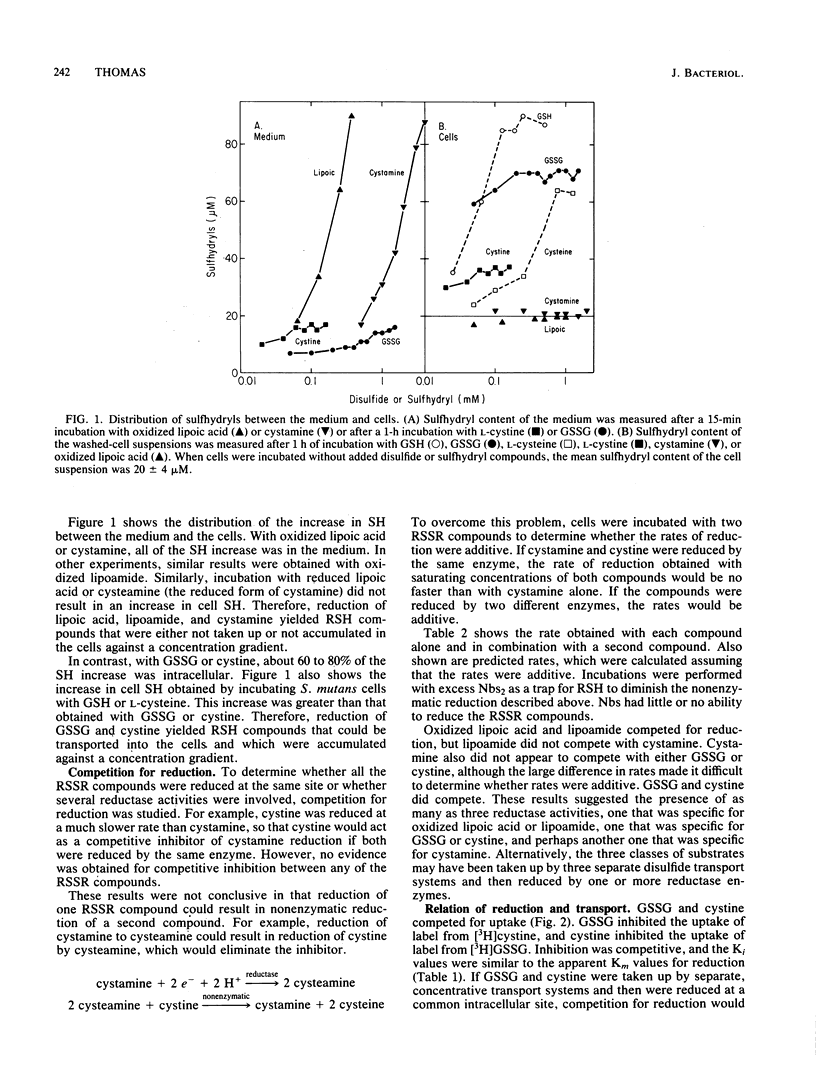
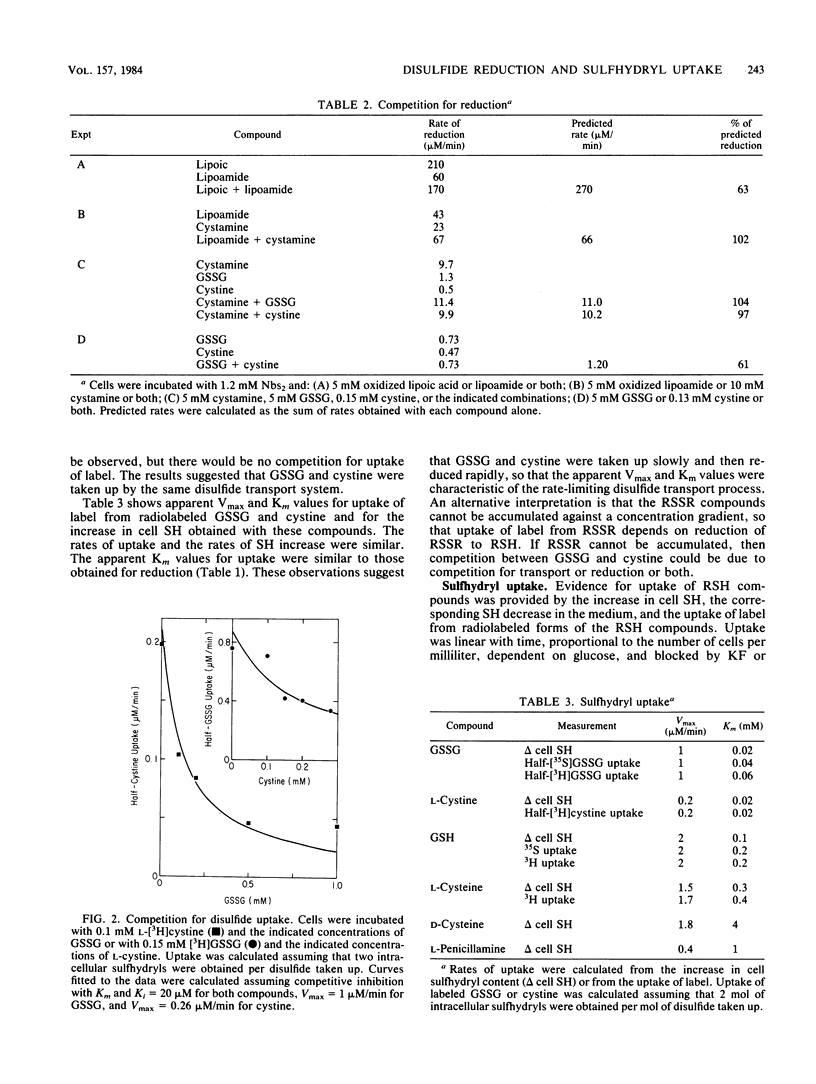
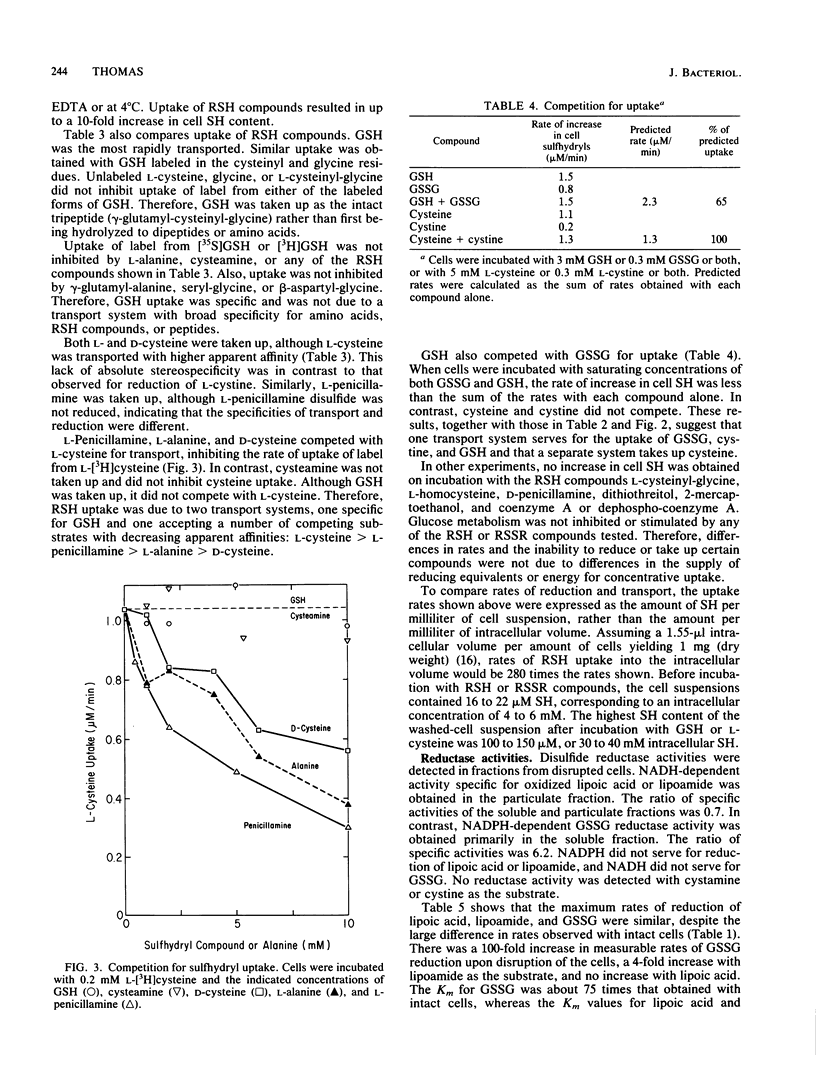
Incubation of Streptococcus mutans cells with certain disulfide compounds resulted in accumulation of reduced sulfhydryl compounds in the extracellular medium or in both the medium and the cells. Oxidized lipoic acid and lipoamide competed for reduction. At high concentrations, these compounds were reduced at rates comparable to that of glucose metabolism, and all of the increase in sulfhydryls was in the medium. Cystamine did not compete with these compounds for reduction but was also reduced at high rates and low apparent affinity, and all of the cysteamine produced from cystamine accumulated in the medium. In contrast, glutathione disulfide (GSSG) and L-cystine were reduced slowly but with high apparent affinity, and 60 to 80% of the increase in sulfhydryls was intracellular. NADH-dependent lipoic acid or lipoamide reductase activity was present in the particulate (wall-plus-membrane) fraction, whereas NADPH-dependent GSSG reductase activity was present in the soluble (cytoplasmic) fraction. Two transport systems for disulfide and sulfhydryl compounds were distinguished. GSSG, L-cystine, and reduced glutathione competed for uptake. L-Cysteine was taken up by a separate system that also accepted L-penicillamine and D-cysteine as substrates. Uptake of glutathione or L-cysteine, or the uptake and reduction of GSSG or L-cystine, resulted in up to a 10-fold increase in cell sulfhydryl content that raised intracellular concentrations to between 30 and 40 mM. These reductase and transport systems enable S. mutans cells to create a reducing environment in both the extracellular medium and the cytoplasm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome M. C., Thomas M. P., Hillier A. J., Jago G. R. Pyruvate dehydrogenase activity in group N streptococci. Aust J Biol Sci. 1980 Mar;33(1):15–25. [PubMed] [Google Scholar]
- Cole J. A. A biochemical approach to the control of dental caries. Biochem Soc Trans. 1977;5(4):1232–1239. doi: 10.1042/bst0051232. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. Oxygen toxicity in Streptococcus sanguis. The relative importance of superoxide and hydroxyl radicals. J Biol Chem. 1982 Apr 25;257(8):4046–4051. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L., Dorian R., Kosower E. M. Analysis of biological thiols: derivatization with monobromotrimethylammoniobimane and characterization by electrophoresis and chromatography. Anal Biochem. 1980 Sep 1;107(1):1–10. doi: 10.1016/0003-2697(80)90483-2. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon C. B., Klebanoff S. J. A peroxidase-mediated, streptococcus mitis-dependent antimicrobial system in saliva. J Exp Med. 1973 Feb 1;137(2):438–450. doi: 10.1084/jem.137.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewen P. C. Levels of glutathione in Escherichia coli. Can J Biochem. 1979 Feb;57(2):107–111. doi: 10.1139/o79-013. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh S. Y., Knowles C. J. Growth of Streptococcus faecalis var. zymogenes on glycerol: the effect of aerobic and anaerobic growth in the presence and absence of haematin on enzyme synthesis. J Gen Microbiol. 1982 May;128(5):1009–1017. doi: 10.1099/00221287-128-5-1009. [DOI] [PubMed] [Google Scholar]
- Swerdlow R. D., Setlow P. Purification and characterization of a Bacillus megaterium disulfide reductase specific for disulfides containing pantethine 4',4"-diphosphate. J Bacteriol. 1983 Jan;153(1):475–484. doi: 10.1128/jb.153.1.475-484.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Tenovuo J., Valtakoski J. The correlation between salivary peroxidase activity, salivary flow rate, and the oxidation-reduction potentials of human saliva and dental plaque suspensions. Acta Odontol Scand. 1976;34(3):169–176. doi: 10.3109/00016357609002565. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob Agents Chemother. 1978 Jun;13(6):1006–1010. doi: 10.1128/aac.13.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Peroxidase antimicrobial system of human saliva: requirements for accumulation of hypothiocyanite. J Dent Res. 1981 Apr;60(4):785–796. doi: 10.1177/00220345810600040401. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


