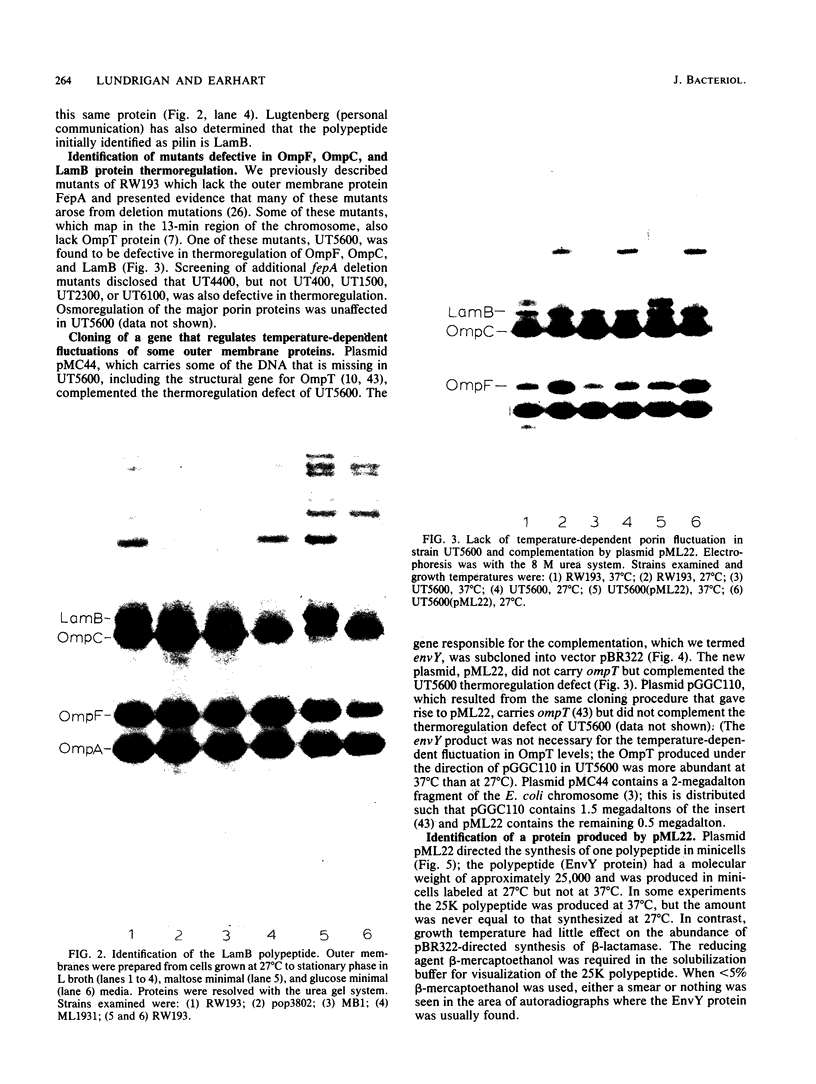
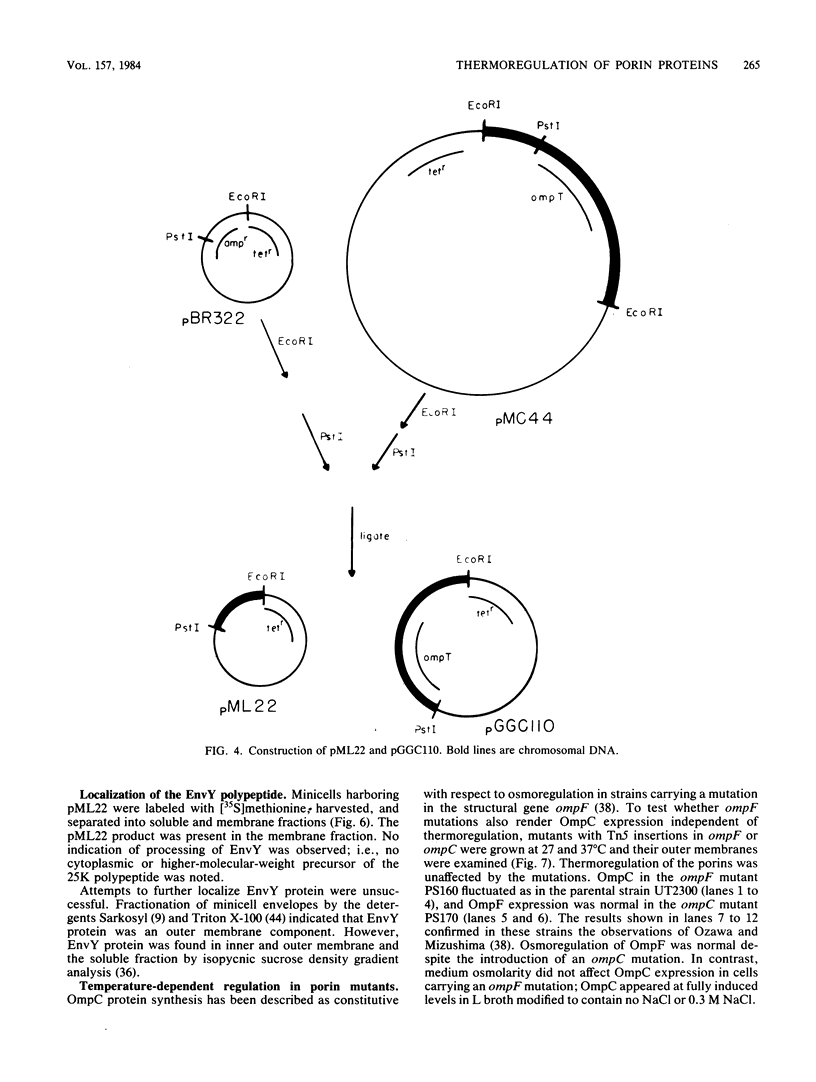
Abstract
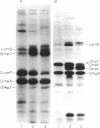
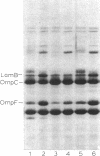
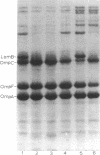
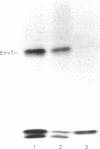
The temperature-dependent expression of OmpF and OmpC, the major channel-forming proteins of the Escherichia coli K-12 outer membrane, was studied. In wild-type cells, decreasing growth temperatures resulted in increased amounts of OmpF protein and correspondingly decreased quantities of OmpC protein. Bacteria deleted for the 13-min chromosomal region did not exhibit this temperature-dependent fluctuation in porin proteins. Plasmid pML22, which consists of pBR322 containing a 0.5-megadalton E. coli chromosomal DNA insert, complemented the thermoregulatory defect. The regulatory gene was named envY. In minicells, pML22 directed the synthesis of an envelope polypeptide (EnvY) having an apparent molecular weight of 25,000. The EnvY protein was synthesized in minicells in greater amounts at 27 degrees C than at 37 degrees C, and a reducing agent was necessary in the solubilization buffer for its subsequent detection on polyacrylamide gels. The results describe the initial characterization of a regulatory system which, along with proteins of the ompB operon, the cyclic AMP system, and the tolC gene product, is involved in a complex network affecting major porin expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P. E., Gayda R., Avni H., Zehnbauer B., Markovitz A. Cloning of Escherichia coli DNA that controls cell division and capsular polysaccharide synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):697–701. doi: 10.1073/pnas.73.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda R. C., Markovitz A. Cloned DNA fragment specifying major outer membrane protein a in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):369–380. doi: 10.1128/jb.136.1.369-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Mättänen P. L., Palva E. T. Cloning of the regulatory locus ompB of Salmonella typhimurium LT-2. II. Identification of the envZ gene product, a protein involved in the expression of the porin proteins. Mol Gen Genet. 1982;188(2):190–194. doi: 10.1007/BF00332674. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Lancaster J. H., Earhart C. F. UC-1, a new bacteriophage that uses the tonA polypeptide as its receptor. J Virol. 1983 Feb;45(2):700–707. doi: 10.1128/jvi.45.2.700-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M., Earhart C. F. Reduction in three iron-regulated outer membrane proteins and protein a by the Escherichia coli K-12 perA mutation. J Bacteriol. 1981 May;146(2):804–807. doi: 10.1128/jb.146.2.804-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M. A., Chenault S. S., Earhart C. F. Genetic and physiological studies on the relationship between colicin B resistance and ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):653–657. doi: 10.1128/jb.137.1.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977 Jul;131(1):331–339. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M. A., Pickett C. L., Chenault S. S., Earhart C. F. Suppression of iron uptake deficiency in Escherichia coli K-12 by loss of two major outer membrane proteins. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1106–1112. doi: 10.1016/0006-291x(78)91250-0. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Mizuno T., Wurtzel E. T., Inouye M. Osmoregulation of gene expression. II. DNA sequence of the envZ gene of the ompB operon of Escherichia coli and characterization of its gene product. J Biol Chem. 1982 Nov 25;257(22):13692–13698. [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Ozawa Y., Mizushima S. Regulation of outer membrane porin protein synthesis in Escherichia coli K-12: ompF regulates the expression of ompC. J Bacteriol. 1983 May;154(2):669–675. doi: 10.1128/jb.154.2.669-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Rupprecht K. R., Gordon G., Lundrigan M., Gayda R. C., Markovitz A., Earhart C. omp T: Escherichia coli K-12 structural gene for protein a (3b). J Bacteriol. 1983 Feb;153(2):1104–1106. doi: 10.1128/jb.153.2.1104-1106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel E. T., Chou M. Y., Inouye M. Osmoregulation of gene expression. I. DNA sequence of the ompR gene of the ompB operon of Escherichia coli and characterization of its gene product. J Biol Chem. 1982 Nov 25;257(22):13685–13691. [PubMed] [Google Scholar]