Abstract
Plant cells are considered to possess functionally different types of vacuoles in the same cell. One of the papers cited in support of this concept reported that protein storage and lytic vacuoles in root tips of barley (Hordeum vulgare) and pea (Pisum sativum) seedlings were initially separate compartments that later fused to form a central vacuole during cell elongation. We have reinvestigated the situation in these two roots using immunogold electron microscopy as well as immunofluorescence microscopy of histological sections. Using antisera generated against the whole protein of α-tonoplast intrinsic protein (TIP) as well as specific C-terminal TIP peptide antisera against α-, γ-, and δ-TIP, together with antisera against the storage proteins barley lectin and pea legumin and vicilin, we were unable to obtain evidence for separate vacuole populations. Instead, our observations point to the formation of a single type of vacuole in cells differentiating both proximally and distally from the root meristem. This is a hybrid-type vacuole containing storage proteins and having both α- and γ-TIPs, but not δ-TIP, in its tonoplast. As cells differentiate toward the zone of elongation, their vacuoles are characterized by increasing amounts of γ-TIP and decreasing amounts of α-TIP.
Most differentiated plant cells are dominated by the vacuole, an organelle that, by storing sugars, inorganic ions, organic acids, and secondary metabolites, serves to generate and regulate cell turgor (De, 2000). The various functions of the vacuole are highly tissue specific (Marty, 1999; Tomos et al., 2000), whereby a distinction is generally made between lytic vacuoles (LVs), which harbor hydrolytic enzymes, and protein storage vacuoles (PSVs), in which nonenzymic proteins accumulate. PSVs are, however, compound organelles because they contain specific hydrolytic enzymes required for storage protein processing (Müntz and Shutov, 2002), as well as crystalline inclusions (Jiang et al., 2000). LVs also have vacuolar processing enzymes, but these belong to a genetically different class of proteins than those present in the PSVs (Hara-Nishimura and Maeshima, 2000). As might be expected, PSVs are usually found in organs where proteins are stored in large amounts (e.g. seeds, especially of legumes; Pernollet, 1978; Bewley and Black, 1994). However, in response to wounding (depodding), storage proteins may also accumulate in vacuoles in vegetative tissues (Franceschi et al., 1983; Staswick, 1994). More recently, PSV-type vacuoles have also been detected in mesophyll cells (Di Sansebastiano et al., 1998; Park et al., 2004).
In addition to lumenal content, vacuoles may also be distinguished from one another on the basis of specific isoforms of tonoplast intrinsic proteins (TIPs; Johnson et al., 1989; Höfte et al., 1992; Chrispeels et al., 1997), which belong to the superfamily of aquaporins (Preston et al., 1992; Chrispeels and Maurel, 1994). Thus, α-TIP is present in the tonoplast of the PSVs in bean (Phaseolus vulgaris) seeds (Johnson et al., 1989), whereas γ-TIP is typical of LVs and is highly expressed in elongating Arabidopsis (Arabidopsis thaliana) root cells (Ludevid et al., 1992). Polyclonal antisera generated against complete TIP molecules have been successfully used on numerous occasions to identify LV and PSV membranes in subcellular fractions by western blotting (Hoh et al., 1995; Barrieu et al., 1998) and in situ by immunogold electron microscopy (IEM; Hoh et al., 1995; Fleurat-Lessard et al., 1997, 2005; Barrieu et al., 1998; Serraj et al., 1998; Hinz et al., 1999). More recently, antibodies raised against specific peptides at the nonconserved C-tail of the TIP molecule (Jauh et al., 1999; Jiang et al., 2001) have also proved to be of diagnostic value.
A number of studies have shown that different types of vacuoles can coexist in the same cell. Some of these rely on obvious visible differences in the lumen of the vacuoles in the electron microscope (e.g. Fleurat-Lessard, 1988; Hoh et al., 1995). In other investigations, variable accumulation of expressed GFP-tagged soluble vacuolar markers (aleurain-GFP for LV-type vacuoles; GFP-chitinase for PSV-type vacuoles) has been used as an indicator for different populations of vacuoles (Di Sansebastiano et al., 1998, 2001). Interestingly, however, targeting of a particular soluble vacuolar marker to the large central vacuole or to smaller vacuoles in the cell cortex seems to vary from cell type to cell type, especially in leaves (Flückiger et al., 2003). These differences may well relate to vacuolar pH with some vacuoles being neutral and others acidic (Swanson et al., 1998; Diwu et al., 1999; Epimashko et al., 2004).
The most frequently cited paper in support of what is sometimes termed the two-vacuole hypothesis is a paper from Paris et al. (1996), which deals with the identification of different vacuole populations in root tips. Because roots have meristems whose cells have no or only small vacuoles, followed distally by a zone of elongation that is functionally correlated with vacuolar expansion, this is a classical organ for study on vacuole biogenesis and vacuole development (Marty, 1999; De, 2000). Until publication of the paper by Paris et al. (1996), vacuoles in the primary root, being vegetative tissue, were generally considered to be of the LV type. Using α- and γ-TIP antisera as membrane probes and antibodies against barley lectin and aleurain as content markers, Paris et al. (1996) demonstrated the presence of PSVs in addition to LVs in the roots of barley and pea. According to these authors, the two vacuole types were separate compartments in the immediate postmeristematic cells and later fused with one another to form the large central vacuole as the cells differentiate upward in the root.
We have reinvestigated the distribution of TIPs and content markers in the roots of barley and pea using immunofluorescent-labeled sections from Steedman wax-embedded samples and IEM. In contrast to Paris et al. (1996), whose observations were made on squash (Cucurbita pepo) preparations where cell position in the root cannot be accurately determined, our results do not support the existence of two initially separate vacuole populations. Instead, postmeristematic cells both in the calyptra and upward in the root form only one type of vacuole: a PSV. As differentiation proceeds, αTIP is gradually replaced by γ-TIP in the membrane of the enlarging vacuole. Production of a PSV rather than a LV as the primary vacuole type in postmeristematic cells is most likely related to seed-specific signals still being dominant in the meristem of the primary root.
RESULTS
Detection of Storage Proteins and TIPs in Barley and Pea Roots
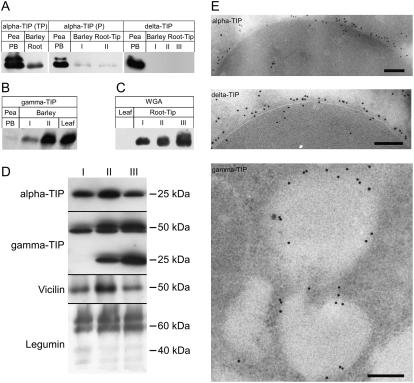
We isolated total membrane fractions from 1-mm segments sequentially excised from barley and pea root tips, separated the proteins by SDS-PAGE, and carried out western blotting with TIP antisera and antibodies against barley lectin (for barley roots) and vicilin and legumin (for pea roots). In barley roots, both α- and γ-TIPs were found to be present in all root tip segments (Fig. 1, A and B). For the detection of α-TIP, polyclonal antibodies directed against the whole protein (Johnson et al., 1989) as well as the C-terminal peptide (Jauh et al., 1998, 1999) were used. Similarly, for γ-TIP, a peptide antibody (Fig. 1B; Jauh et al., 1998) and a whole protein antibody (VM 23; Maeshima, 1992; data not shown) were used and gave identical results. However, δ-TIP, which, together with α-TIP, is typical of the boundary membrane of protein bodies from pea cotyledons (Fig. 1E), was not detected by the δ-TIP peptide antiserum of Jauh et al. (1998) in barley root tips. Barley lectin was also found to be present in all root tip segments (Fig. 1C). α- and γ-TIPs were detected in all segments of the pea root tip (Fig. 1E), as were vicilin and legumin—the principal storage globulins of pea seeds. Interestingly, these globulins were not proteolytically processed into mature storage proteins as they are in developing cotyledons. Thus, only prolegumin polypeptides with molecular mass around 60 kD (Hinz et al., 1999) were detected in root tips of pea.
Figure 1.
Immunological detection of TIPs. A, α-TIP, but not δ-TIP, is present in membranes extracted from 1-mm segments excised from root tips of 3-d-old barley seedlings. As controls for PSV membranes, a protein body fraction was isolated from pea seeds. Two types of α-TIP antisera were used: against an α-TIP total protein (Johnson et al., 1989) and against a C-terminal peptide (Jauh et al., 1999). B, γ-TIP is also present in membranes from barley root tips. Again, two different antisera (γ-TIP antibodies generated against a C-terminal peptide [Jauh et al., 1998]; γ-TIP [=VM23] total protein antibodies [Maeshima, 1992]) gave the same result (data not shown). A membrane fraction isolated from barley leaves is used as a control for LV membranes. C, Barley lectin (=wheat germ agglutinin [WGA]) is present in root tips, but not leaves of barley. D, Detection of TIPs and unprocessed forms of storage globulins in 1-mm root tip segments from 3-d-old pea seedlings. Note that legumin, when processed, has two polypeptides at 40 and 20 kD (linked by a disulfide bridge). Our antiserum (Hinz et al., 1999) recognizes only the 60-kD precursor and the 40-kD mature polypeptide, which are shown in the blot, but not the 20-kD polypeptide. E, Controls for immunogold detection of TIPs; pea cotyledon protein bodies for α- and δ-TIPs; vacuoles in elongation zone of barley roots for γ-TIP. Cryosections are labeled with peptide antisera (Jauh et al., 1998). Bars = 100 nm.
Cellular Distribution of Barley Lectin and TIPs in Barley Roots
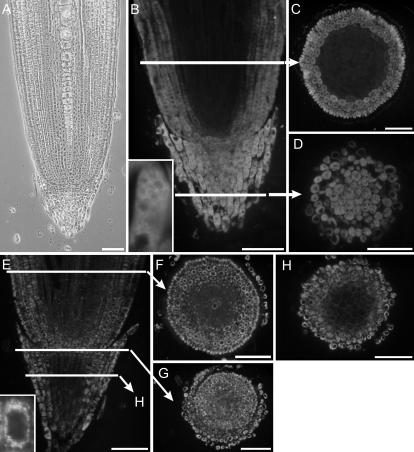
Barley lectin is not uniformly expressed throughout the root tips of germinating barley, neither in 3- nor in 10-d-old roots (Fig. 2, B–D; Supplemental Fig. 1A). Whereas all cells of the calyptra stained positively for barley lectin (Fig. 2, B–D), its synthesis in the rest of the root tip is restricted to the rhizodermis and the outermost two to three cell layers of the cortex (Fig. 2, B and C; Supplemental Fig. 1A). No lectin signal was visible in the cells of the central cylinder. Interestingly, whereas the immunostaining pattern for α-TIP was similar to that for barley lectin in the non-calyptra portion of the root tip, only the cells surrounding the columella of the calyptra stained positively for α-TIP (Fig. 2, E–H). In root tips from older seedlings, the α-TIP signal is particularly strong in the cells of the meristem (Supplemental Fig. 1B). In addition, cells of the central cylinder are also labeled, but more weakly. Distribution of γ-TIP in 3-d-old roots was remarkably similar to that of barley lectin, with all cells of the calyptra and only the rhizodermis and outermost cortex cells reacting positively (Fig. 3, A–C). In comparison, in 10-d-old roots, cells of the central cylinder gave a weak signal for γ-TIP, but there was almost no signal in the rhizodermis (Supplemental Fig. 1C).
Figure 2.
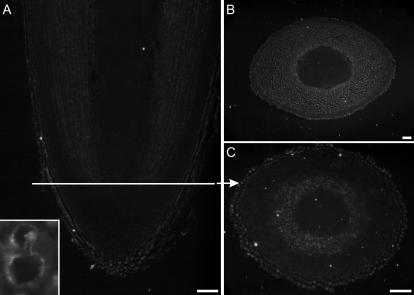
Localization of barley lectin and α-TIP in root tips of 3-d-old barley seedlings. A, Phase contrast picture of longitudinal section. B to D, Lectin is found in the cells of the calyptra, the rhizodermis, and the outermost cells of the cortex. Inset, High magnification of a single calyptra cell. The lectin signal is more intense at the rims of the vacuoles, as seen in EM sections (Fig. 6). E to H, α-TIP has a similar distribution to barley lectin, except that it is absent from the innermost cells of the calyptra. E and H, α-TIP total protein antiserum (Johnson et al., 1989). G, α-TIP peptide antiserum (Jauh et al., 1998). Arrowhead points to the meristem. Bars = 100 μm.
Figure 3.
Localization of γ-TIP in root tips of 3-d-old barley seedlings with γ-TIP peptide antiserum (Suga and Maeshima, 2004). The distribution of γ-TIP is similar to that of barley lectin and is restricted to the calyptra, rhizodermis, and outermost cortical cells. A, Longitudinal section; B, transverse section above the meristem; C, transverse section through the calyptra. Bars = 50 μm.
Cellular Distribution of Vicilin and TIPs in Pea Roots
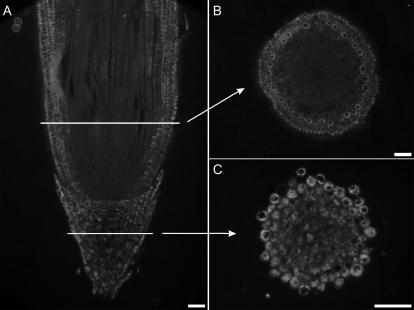
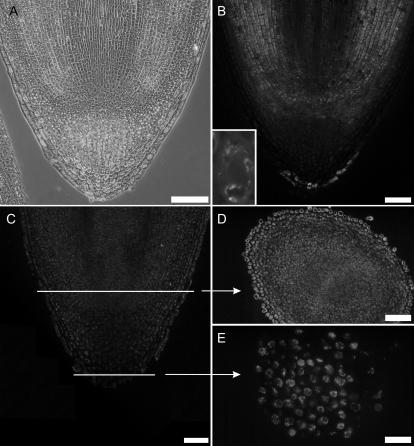
In root tips of 3-d-old pea seedlings, labeling with vicilin antibodies was seen in the meristem, cortex, and extreme tip of the calyptra, but neither in the central cylinder nor the rest of the calyptra (Fig. 4B). In 10-d-old root tips, vicilin and α-TIP were no longer detected (Supplemental Fig. 1, D and E). In pea root tips, γ-TIP labeling was observed in the outermost cells of the root cap and the innermost layers of the cortex above the meristem, whereas the meristem itself, the majority of the calyptra, and the rhizodermis were without signal (Fig. 5, A–C). In 10-d-old root tips, the γ-TIP signal resembled that of 3-d-old root tips, with the strongest signal being in the innermost cells of the cortex (Supplemental Fig. 1F).
Figure 4.
Localization of vicilin and α-TIP in root tips of 3-d-old pea seedlings. A, Phase contrast picture of longitudinal section. B, Vicilin is present only at the outermost cells of the calyptra, but also in the innermost cells of the cortex. Inset, High magnification of a single cell at the flank of the meristem revealing peripheral vicilin deposits in vacuoles. C to E, α-TIP is expressed by nearly all cells of the root tip except columella cells. α-TIP was detected by α-TIP total protein antiserum (Johnson et al., 1989); the same results were obtained for α-TIP peptide antibodies (Jauh et al., 1998); data not shown. Bars = 100 μm (A and B); 50 μm (C–E).
Figure 5.
Localization of γ-TIP in root tips of 3-d-old pea seedlings. γ-TIP is strongly expressed by the innermost cells of the cortex, but is lacking from the central cylinder and rhizodermis. The root cap shows a γ-TIP signal that is more pronounced in the outer cell layers. A, Longitudinal section. Inset, High magnification of a single cell from the cortex shows γ-TIP labeling is restricted to the periphery of vacuoles. B and C, Transverse sections in regions above (B) and in the region of the meristem (C). Bars = 100 μm.
IEM of Barley and Pea Root Tips: No Evidence for Separate Vacuole Populations
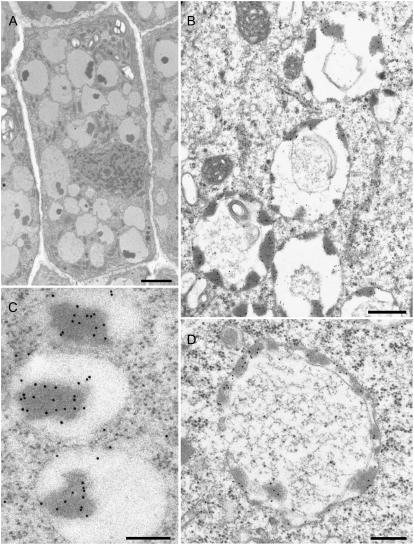
We have performed IEM on barley root tips with antibodies generated against the storage protein barley lectin as a content marker, and with antibodies against α- and γ-TIP. We selected cells in those areas that stained positively for barley lectin by immunofluorescence: cells immediately bordering on the meristem, cells in the cortex on the flanks, and cells in the calyptra. The vacuoles in all cells investigated had electron opaque deposits, which stained positively with barley lectin antibodies (Fig. 6, A–D). In cells in the cortical flank (Fig. 6C) or in the calyptra (Fig. 6A), large lectin-positive deposits, sometimes in the middle of the vacuole lumen, were regularly observed. In cells immediately surrounding the quiescent center where vacuole formation begins, we observed vacuoles with internal membranes in addition to lectin-positive protein aggregates (Fig. 6, B–D). Such structures are typical for vacuoles that arise from an initially cisternal-tubular system by a progressive, inwardly directed, dilation, as previously described for PSVs in developing pea cotyledons (Hoh et al., 1995). An early stage in this process, where an almost complete tire-like provacuole with protein aggregates is seen surrounding what is sometimes termed a zone of exclusion (Amelunxen and Heinze, 1984; Robinson and Hinz, 1997), is presented in Figure 6D.
Figure 6.
Immunogold detection of barley lectin in vacuoles of 3-d-old barley roots. A, Cell in the calyptra revealing electron opaque aggregates within the vacuoles. B to D, Electron opaque aggregates stain positively with anti-wheat germ agglutinin gold (10 nm) conjugates. C and D, Stages in vacuole formation in cells immediately bordering on the meristem. Barley lectin-positive aggregates are positioned more on the outer than the inner tonoplast membrane. Bars = 1.5 μm (A), 400 nm (B), 150 nm (C), 300 nm (D).
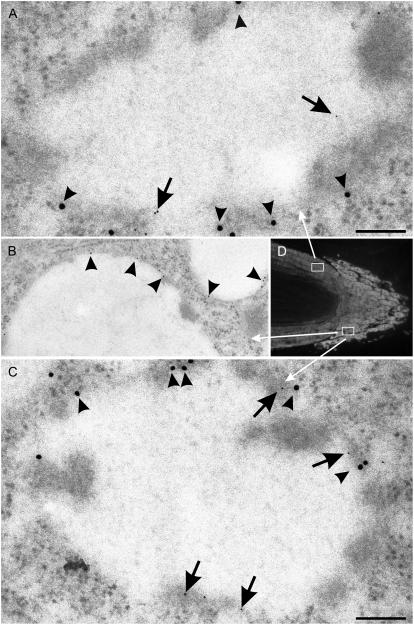
Vacuoles with storage protein aggregates in barley roots stained positively with γ-TIP antibodies in single immunogold labeling preparations (Fig. 7B). We also performed a variant of double immunogold labeling by which different sides of the section were exposed to a different antibody. In this way, we were able to demonstrate the presence of both α- and γ-TIPs in the membrane of PSVs present in cells from the cortical flank (Fig. 7, A and D) and the calyptra (Fig. 7, C and D).
Figure 7.
Immunogold detection of TIPs in cells from 3-d-old barley roots. A and C, Double immunogold labeling with α-TIP (15-nm gold particles, arrowheads; α-TIP peptide antiserum [Jauh et al., 1998]) and γ-TIP (5-nm gold, arrows; γ-TIP peptide antiserum [Jauh et al., 1998]). B, Single immunogold labeling of a PSV (note storage protein aggregates on the tonoplast) with γ-TIP peptide antiserum (10-nm gold [Jauh et al., 1998]). D, Longitudinal section of root for cell positioning. Boxes indicate areas in the cortex and calyptra from which the vacuoles in A and C are depicted. Bars = 100 nm (A and C); 200 nm (B).
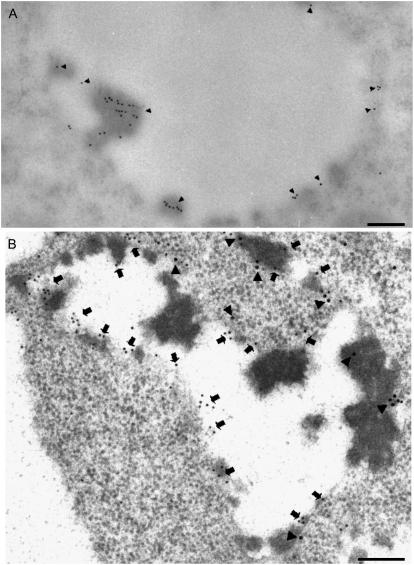
Vacuoles from cells in the cortical flanks distal to the meristem of pea roots, which reacted positively toward vicilin antibodies in immunofluorescence, also contained vicilin-positive storage protein aggregates (Fig. 8A). These vacuoles also labeled positively with both α- and γ-TIP antibodies (Fig. 8B). In double immunogold-labeled sections, we could not find vacuoles that were labeled exclusively by only one TIP antibody.
Figure 8.
Immunogold detection of vicilin and TIPs in 3-d-old root tips of pea seedlings. A, Vicilin-positive immunogold labeling (10-nm gold particles) of a vacuole from the inner cortex; cryosections. B, Double immunogold labeling with α-TIP (15-nm gold, arrowheads; α-TIP peptide antiserum [Jauh et al., 1998]) and γ-TIP (5-nm gold, arrows; γ-TIP peptide antiserum [Jauh et al., 1998]) of a PSV from the same cell type. Bars = 150 nm (A); 200 nm (B).
DISCUSSION
The classical notion of the vacuole as being a multifunctional single organelle in the plant cell (Wink, 1993) has gradually been superseded over the last decade by a multicompartment concept with different vacuole types coexisting in the same cell (Robinson and Rogers, 2000). Not only has convincing morphological data been presented for functionally distinct vacuoles (Epimashko et al., 2004; Neuhaus and Paris, 2006), but indirect evidence for the operation of different transport routes for storage proteins and acid hydrolases also exists (Vitale and Hinz, 2005; Hinz et al., 2007; Sanmartin et al., 2007). Especially important for the development of the multivacuolar concept has been the discovery of vacuole-specific isoforms of TIPs (Johnson et al., 1989; Höfte et al., 1992; Ludevid et al., 1992). These allow lytic-type vacuoles to be distinguished from vacuoles in which proteins are stored, especially in seeds. Actually, the TIP isoforms are very similar and differ only in their C-terminal cytoplasmic tails against which specific peptide antibodies have been generated (Jauh et al., 1998, 1999). In immunofluorescence studies, together with antibodies against content proteins (e.g. the Cys protease barley aleurain for LVs; Paris et al., 1996; Jauh et al., 1999), vegetative storage proteins and protease inhibitors for vegetative PSVs (Jauh et al., 1998), and barley lectin and chitinase for seed PSVs (Jiang et al., 2000), it has been possible to allocate the TIP isoforms to specific vacuole types. Thus, according to Jauh et al. (1998, 1999), PSVs in vegetative tissues are basically characterized by δ-TIP, whereby seed PSVs may have, in addition, α- or γ-TIP. In contrast, LVs have only γ-TIP, and autolysosomes—induced by starvation or treatment with the Cys protease inhibitor E-64—have only α-TIP in their membranes (Moriyasu et al., 2003).
A key paper in support of the multivacuole concept is that of Paris et al. (1996), which described the existence of two separate populations of vacuoles in root tips of barley and pea seedlings. These two vacuoles, a PSV marked by the presence of barley lectin and α-TIP and a LV with aleurain and γ-TIP, were reported to be present in cells immediately derived from the meristem, but, as cell growth continued, they fused with one another to form a large central vacuole. Unfortunately, the data of Paris et al. (1996) was essentially obtained on individual cells released by enzymatic maceration so that, for all double labeling, the original position of the cells in the root could not be ascertained. Paris et al. (1996) did present immunofluorescent data from histological sections of root tips, but only for single TIPs in different organisms: α-TIP in pea and barley, and γ-TIP in pea. In agreement with their results, we also found that TIPs, in both root tips, are not equally expressed in all tissues, although this does appear to be the case with V-ATPase (Supplemental Fig. S2). In particular, cells in the central cylinder (stele) showed weak or no signals for either α- or γ-TIP, as well as the storage proteins barley lectin and vicilin. This result was confirmed in the electron microscope: Tonoplasts of cells in the central cylinder did not react positively to any of the antisera (data not shown). Exactly what kind of TIP is expressed in the vacuoles of the central cylinder remains a matter for speculation. We should not, however, fail to point out that our observations and those of Paris et al. (1996) stand in contradiction to those of Höfte et al. (1992) because the latter authors presented western blots showing that α-TIP expression was restricted to the embryo and endosperm in Arabidopsis and did not occur in the roots. The reason for this difference is unclear, but it cannot be a question of the antisera (peptide antibodies versus antibodies raised against the total TIP protein) because the original α-TIP antiserum of Johnson et al. (1989), which was used by Höfte et al. (1992), also gave a positive signal in barley roots (see Fig. 1).
The detection of vicilin and legumin, albeit as unprocessed proforms, in the vacuoles of pea root tips is a significant observation. The presence of these seed tissue globulins in nonstorage tissues (i.e. roots) during seed germination seems at first glance surprising. The question is whether this reflects the continued expression of seed globulin genes during germination or merely the presence of residual globulins synthesized during embryogenesis. It has been previously demonstrated that vicilin and legumin are not only expressed during embryogenesis, but also during microsporogenesis (Zakharov et al., 2004), and this has been interpreted as a response to the need for reserves of amino acids required during seed and pollen germination (Hall et al., 1999). However, it has also been shown that promoters for numerous storage globulins are precisely regulated, being highly active during embryogenesis, and then being switched off during all subsequent phases of vegetative development (Chandrasekharan et al., 2003). Therefore, it would seem that the presence of storage proteins in root tip cells of 3-d-old pea seedlings is not a result of new synthesis during germination, especially because they are no longer detectable after 10 d of germination. In this regard, the study of Tiedemann et al. (2000) is worthy of mention. They reported that, during embryogenesis in vetch (Vicia sativa), storage globulins are expressed both in the cotyledons and the embryonic axis (which gives rise to the radicle and subsequently the root). Interestingly, storage globulins in the embryonic axis are consumed during the first 2 to 3 d of germination because mobilization of globulins stored in the cotyledons first sets in after this time. Thus, for the early stages in germination, the radicle/young root is autonomous in terms of amino acid provision. However, this situation and its timing vary considerably from species to species (Tiedemann et al., 2000).
If there is no new synthesis of storage globulins during the growth of the radicle and primary root for the first days of germination, how is it that, after 3 d of germination, storage globulins synthesized during embryogenesis are still distributed in the cells of the root tip, including the cells of the calyptra, which are products of the root apical meristem? If storage globulin synthesis in the embryonic axis/radicle no longer occurs during germination, one would expect an increasingly weaker globulin signal in the newly formed cells of the root tip as it grows away from the seed, but this does not appear to be the case. In addition, if the globulins seen in the young root were merely being carried over out of the embryo, they should be present as processed forms. That they are not is an indication that they were newly synthesized during germination in tissues lacking processing enzymes.
The situation with barley lectin in the vacuoles of the root tip of 3-d-old barley seedlings may be different from the seed storage globulins of pea seedlings. Our IEM investigations confirm the presence of the barley lectin in vacuoles in the root tip of barley. This is not unexpected because other lectins (e.g. phytohemagglutinin) are known to be synthesized in the embryonic axis and root tips of bean seedlings (Greenwood et al., 1984; Kjemtrup et al., 1995) and play roles in defense against pathogens (Chrispeels and Raikhel, 1991) and in Rhizobium recognition (Bohlool and Schmidt, 1974). Unclear, however, is whether the lectin-positive electron opaque deposits in barley root tip vacuoles are made up exclusively of barley lectin or whether other storage proteins contribute to these aggregates. Nevertheless, we have screened sections in and around the meristem, in the calyptra, and in the flank region of the root cortex, principally in barley, but also in pea root tips, and were unable to detect a population of vacuoles that stained exclusively with a single TIP. Double immunogold labeling of sections showed that both α- and γ-TIPs were always present together in the same tonoplast. Although our observations were concentrated on cells in the root tip, in particular in barley, we did notice that cells in the elongation zone had progressively less detectable α-TIP. Thus, in contrast to Paris et al. (1996), who used the same plant materials, our data show that cells arising from the meristem in roots do not develop separate PSV and LV compartments, which ultimately fuse to form a central vacuole. Instead, initially only a single vacuole type is formed, which has mixed PSV and LV characteristics. This may be due to the insertion of newly synthesized γ-TIP into the original PSV creating a hybrid vacuole, which is then gradually transformed into a central vacuole of the LV type as elongation and differentiation proceed.
Our data qualify, but do not revoke, the multicompartment concept for the plant vacuole. Whereas root tips of young barley and pea seedlings do not develop separate populations of PSV- and LV-type vacuoles, the literature (cited above) contains sufficient examples of different vacuole populations in a single cell. Perhaps the most convincing examples are storage parenchyma cells in the cotyledons of developing pea seeds. Here, not only are PSVs from LVs easily distinguishable from one another in sections in the electron microscope, but tonoplast membrane fractions with different isopycnic densities and different TIPs can be isolated from this tissue at different developmental stages (Hoh et al., 1995). The recent paper of Otegui et al. (2006), in which the presence of only a single vacuole during the main phase of storage protein deposition in developing Arabidopsis embryos, might seem to stand in contradiction to the earlier results of Hoh et al. (1995). However, a recent time course analysis of ultrastructural changes during embryo development in Arabidopsis (C. Wie, G. Hinz, and D.G. Robinson, unpublished data) has revealed a series of events closely similar to those described by Hoh et al. (1995) for vacuole biogenesis in pea cotyledons, namely, the de novo development of an initially tubular PSV (not unlike that depicted in Fig. 6D) in the early bent cotyledon stage, which quickly supersedes the previous LV (C. Wie, G. Hinz, and D.G. Robinson, unpublished data).
MATERIALS AND METHODS
Plant Materials
Barley (Hordeum vulgare) var. Madonna (Lochow-Petkus GmbH) and pea (Pisum sativum) var. Kleine Rheinländerin (Wagner) were used in this study. For 3-d-old seedlings, seeds were imbibed for 30 min (barley) or 3 h (pea), placed on moist filter paper, and germinated at room temperature (RT) in the dark. For 10-d-old seedlings, seeds were potted in TKS2 soil (Floragard) after imbibition and grown in a 12-h white fluorescent light (430W Son-T Agro bulbs; Philips) and a 12-h-dark regime at 22°C to 25°C at 40% to 60% relative humidity in a greenhouse.
Antibodies
Antisera and their dilutions used for western blotting (in parentheses) and immunofluorescence (IEM; in brackets) were anti-α-TIP (1:1,000) [1:50] against the C-terminal sequence HQPLAPEDY of Arabidopsis (Arabidopsis thaliana) α-TIP (Jauh et al., 1998); anti-γ-TIP (1:1,000) [1:50–400] against the C-terminal sequence CCSRTHEQLPTTDY of Arabidopsis γ-TIP (Jauh et al., 1998); anti-γ-TIP [1:50] against the C-terminal sequence INQNGHEQPTTDY of radish (Raphanus sativus) VM23 (Suga and Maeshima, 2004); anti-γ-TIP (1:25) against the total VM23 protein isolated from radish (Maeshima, 1992); anti-δ-TIP (1:1,000) [1:50] against the C-terminal sequence HVPLASADF of Arabidopsis δ-TIP (Jauh et al., 1998); anti-V-ATPase [1:50] against the A-polypeptide of the V1 subunit of V-ATPase from Mesembryanthemum crystallinum (Haschke et al., 1989); anti-wheat germ agglutinin (1:1,000) [1:300–400] (Sigma); anti-legumin (1:10,000) [1:50] against pea legumin (Hinz et al., 1999); and anti-vicilin (1:1,000) [1:50–100] against Vicia faba vicilin (Dr. R. Manteufel).
Light Microscopy
Sample Preparation for Immunofluorescence Light Microscopy
Three-millimeter root tips were excised from pea and barley seedlings and fixed in 3.7% (w/v) formaldehyde in PIPES buffer (50 mm PIPES-KOH, pH 6.9, 5 mm MgSO4.7H2O, 5 mm EGTA) for 60 min at RT, then washed in PIPES buffer for 30 min at RT and then in phosphate-buffered saline (PBS; 10 mm K2HPO4/KH2PO4, pH 7.4, 150 mm NaCl) for 30 min at RT. Samples were then dehydrated via an ethanol series: 30% (v/v) ethanol in PBS for 30 min, 50% ethanol in PBS for 30 min, 70% ethanol in PBS for 30 min, and 99% ethanol in PBS for 30 min. Samples were then stained in 0.01% (w/v) toluidine blue in 99% (v/v) ethanol for 5 min at RT and washed in 99% (v/v) ethanol for 5 min.
Wax embedment followed the Steedman procedure (Steedman, 1957) essentially using a wax (90% [w/v]) polyethylene glycol distearate (400 g mol−1, 10% [v/v] 1-hexadecanol) that, after preparation, melts at 37°C. Stained samples in ethanol were added to a similar volume of wax and left to infiltrate overnight at 37°C. On the next day, first the ethanol overlay and then the wax around the samples were removed and new wax was added and incubated for another 2 h before being transferred to RT. Sections (10 μm) were cut with a microtome (Jung) equipped with a steel knife (Leica) and transferred to glass slides previously coated with 10 μL of glycerol albumin. Sections were stretched with water, and, after removing the water, slides were left to dry for at least 15 h. Prior to immunolabeling, wax was removed from the sections through an ethanol series: 99% (v/v) ethanol for 4 × 10 min; 90%(v/v) ethanol in PBS for 10 min; 70% (v/v) ethanol in PBS for 10 min; 50% (v/v) ethanol in PBS for 10 min; 25% (v/v) ethanol in PBS for 10 min; and PBS for 10 min.
Immunofluorescence Labeling
Sections on glass slides were incubated at RT with 100 μL of each of the following: bovine serum albumin (BSA) block solution (1% [w/v] BSA in PBS) for 5 min; Gly block solution (20 mm Gly in PBS) for 5 min; primary antibody diluted in wash buffer (0.1% [w/v] BSA in PBS) for 1 h; wash buffer for 3 × 10 min; secondary antibody (Alexa Fluor 488 goat-anti-rabbit IgG [H + L]) 2 mg mL−1 (Molecular Probes) 1:50 with wash buffer for 1 h; wash buffer for 3 × 5 min; and then PBS for 3 × 5 min. A covering solution was prepared by dissolving 100 mg of p-phenylenediamine in 10 mL of PBS, then 90 mL of glycerol was added and the solution was brought to pH 8.0 with Tris. A drop of the cover solution was added to the labeled sections before they were mounted and sealed with nail varnish.
Examination of the immunofluorescence labeling followed in an Axiovert 200 microscope (Zeiss) using the FT 510 filter (Zeiss) emitting light at 450 to 490 nm and 515 to 565 nm. Images were captured with a Canon G2 digital camera.
IEM
Sample Preparation
Pea and barley root tips as above were fixed in 1.5% paraformaldehyde, 0.25% glutaraldehyde in wash buffer (0.1 m K2HPO4/KH2PO4, pH 7.0) first for 30 min at RT, then overnight at 4°C. Samples were dehydrated through an ethanol series (30% [v/v] ethanol in water for 30 min at 4°C; 50% [v/v] ethanol for 15 min at 4°C, then for 45 min at −20°C; 70% [v/v] for 60 min at −20°C; 100% [v/v] for 2 × 60 min at −20°C) using the progressive lowering of temperature technique in an automatic freeze substitution unit (Leica). Samples were then embedded in HM20 (Lowicryl HM kit; Polyscience; 25% [v/v] HM20 in ethanol for 45 min at −20°C; 50% [v/v] HM20 in ethanol for 45 min at −20°C; 75% [v/v] HM20 in ethanol for 45 min at −20°C; 100% [v/v] HM20 for 1.5 h at −35°C; 100% [v/v] HM20 for 4 h at −35°C) and polymerized in UV light at −35°C. Ninety-nanometer-thick sections were cut with an Ultracut S microtome (Reichert) and transferred to Formvar-coated nickel grids.
Single-Antibody Labeling
HM20-embedded sections on grids were blocked with 3% (w/v) BSA in PBS (10 mm K2HPO4/KH2PO4, pH 7.4, 150 mm NaCl) for 30 min, incubated in primary antibody for 1 h, washed in washing buffer (1% [w/v] BSA in PBS) for 3 × 10 min, incubated with secondary antibody (gold-conjugated goat-anti-rabbit IgG with 5-, 10-, or 15-nm gold particles [EM.GAR 5, 10, or 15; British BioCell International]), diluted 1:50 with PBS for 1 h, and washed in washing buffer for 2 × 5 min and in water for 3 × 5 min.
Double-Antibody Labeling
Because all primary antisera were generated in rabbits, a double-labeling procedure was performed by first gently placing an HM20-embedded section, directly after cutting, onto a drop of blocking solution. Most of the drop was removed afterward with a fine syringe and the next solution added by carefully underlayering it, still allowing the section to float. After the incubation and washing regime (described below), the section was gently transferred to a Formvar-coated grid by sliding the grid under the section so that the upper (nonlabeled) side of the section was now accessible for a new round of labeling with another primary antibody (also as below). Labeling was done using similar solutions as above: blocking in block buffer for 1 × 2 min, then for 1 × 30 min, primary antibody incubation for 1 h, washing in wash buffer 1 × 2 min, then for 3 × 10 min, secondary antibody incubation for 1 h, and washing in wash buffer for 3 × 5 min and in water for 3 × 5 min. The sections were poststained in 3% (w/v) uranyl-acetate in water for 5 min, washed in water for 3 × 1 min, then incubated in 0.3% (w/v) lead citrate in 1 m NaOH for 5 min, washed in water for 4 × 1 min. After drying, samples were examined in a Philips CM10 transmission electron microscope.
Cryosectioning
Cryosections were prepared and labeled with primary and secondary antibodies exactly as described by Pimpl et al. (2000).
Protein Extraction, SDS-PAGE, and Western Blotting
Total Membrane Protein Fractions from Barley and Pea Roots
Three-millimeter root tips were cut and homogenized with acid-washed sea sand in a 10-fold volume of homogenization buffer (40 mm HEPES-KOH, pH 7.0, 300 mm Suc, 10 mm KCl, 3 mm MgCl2), including protease inhibitors (1 mm dithiothreitol, 2 μg mL−1 aprotinin, 0.5 μg mL−1 leupeptin, 2 μg mL−1 pepstatin, 2 mm o-phenanthroline, 1 μg mL−1 E-64). The homogenate was passed through one layer of Miracloth and centrifuged at 1,000g in a Sorvall HB4 rotor for 10 min. The pellet was discarded and the supernatant was centrifuged at 12,000g for 20 min in the same rotor. The pellet was saved and the supernatant centrifuged at 100,000g for 1 h in a Sorvall TFT 50.38 rotor. The supernatant was discarded and the pellets were resuspended in homogenization buffer using a glass homogenizer. Protein concentrations were measured according to Lowry (1951) and protein samples for SDS-PAGE were precipitated with methanol-chloroform (Wessel and Flügge, 1984).
Isolation of Protein Bodies from Pea Cotyledons
Pea seeds were harvested 4 weeks after flowering, the testa removed, and seeds weighed. The isolation procedure was according to Hohl et al. (1996) and Hinz et al. (1999). Essentially, 2 volumes of homogenization buffer (0.1 m MOPS-KOH, pH 5.5, 0.6 m sorbitol, 1 mm EDTA) with antiproteases as above were added and plant material was finely chopped by hand with a razor blade. The homogenate was filtered through 1× Miracloth and centrifuged at 90g in a Labofuge I swing-out rotor for 1 min. The supernatant was loaded onto a 5% (w/v) Ficoll 400 cushion and centrifuged at 460g in a Sorvall HB4 rotor for 10 min. The pellet (material collected from the top of the cushion) was resuspended in homogenization buffer and recentrifuged; this step was then repeated once more. The pellet was saved; protein concentration determination and sample preparation for SDS-PAGE were done as described above.
Isolation of Integral Membrane Proteins from Barley Leaves
Leaves from 5-d-old barley seedlings were homogenized with acid-washed sea sand (40 mm HEPES/KOH, pH 7.0, 300 mm Suc, 10 mm KCl, 3 mm MgCl) with antiproteases as above. The homogenate was passed through 1× Miracloth and centrifuged at 18,000g in a Sorvall HB4 rotor for 20 min. The pellet was discarded and the supernatant was centrifuged at 100,000g in a Sorvall TFT 50.38 rotor for 1 h. The pellet was resuspended in 2 mL of homogenization buffer and diluted 1:10 with potassium iodine solution (1 m KI in 20 mm MES-KOH, pH 7.0), incubated under rotation for 30 min at 4°C to remove peripheral membrane proteins. The solution was then centrifuged again at 100,000g for 1 h and the pellet was resuspended in 20 mm MES-KOH, pH 7.0, and prepared for SDS-PAGE as described above.
Western Blotting
After electrophoretic separation on 10% to 20% polyacrylamide gradient gels using standard procedures, proteins were transferred to nitrocellulose membranes (Pall), blocked in 5% (w/v) milk powder in Tris-buffered saline plus Tween (50 mm Tris-HCl, pH 7.5, 200 mm NaCl, 0.05% [v/v] Tween 20), and incubated with antisera diluted in Tris-buffered saline with 1% (w/v) BSA. Washing procedures, secondary horseradish peroxidase-coupled antibody incubation, and detection of luminescence signals followed essentially the instructions of the SuperSignal West Pico chemiluminescence kit (Pierce).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Immunological detection of storage proteins (barley lectin, vicilin) and TIPs in root tips of 10-d-old barley (A–C) and pea (D–F) seedlings.
Supplemental Figure S2. Immunological detection of V-ATPase in root tips of 3-d-old barley (A) and pea (B) seedlings.
Supplementary Material
This work was supported by the German Research Council (Deutsch Forschungsgemeinschaft Ro 440/13–3) and the Landesgraduiertenförderung of Baden-Württemberg (stipend to A.O.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David G. Robinson (david.robinson@urz.uni-heidelberg.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amelunxen F, Heinze U (1984) Zur Entwicklung der Vakuole in Testa-Zellen des Leinsamens. Eur J Cell Biol 35 343–354 [Google Scholar]
- Barrieu F, Thomas D, Marty-Mazars D, Marty F (1998) Tonoplast intrinsic protein from cauliflower (Brassica oleracea L. var. botrytis): immunological analysis, cDNA cloning and evidence for expression in meristematic tissues. Planta 204 335–344 [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds. Physiology of Development and Germination, Ed 2. Plenum Press, New York
- Bohlool BB, Schmidt E (1974) Lectins—possible basis for specificity in rhizobium-legume root nodule symbiosis. Science 185 269–271 [DOI] [PubMed] [Google Scholar]
- Chandrasekharan MB, Bishop KJ, Hall TC (2003) Module-specific regulation of the β-phaseolin promoter during embryogenesis. Plant J 33 853–866 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Daniels MJ, Weig A (1997) Aquaporins and water transport across the tonoplast. Adv Bot Res 25 419–432 [Google Scholar]
- Chrispeels MJ, Maurel C (1994) Aquaporins—the molecular basis of facilitated water movement through living plant cells. Plant Physiol 105 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Raikhel NV (1991) Lectins, lectin genes, and their role in plant defense. Plant Cell 3 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De DN (2000) Plant Cell Vacuoles. An Introduction. CSIRO Publishing, Collingwood, Australia
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (1998) Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J 15 449–457 [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (2001) Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuole. Plant Physiol 126 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwu ZJ, Chen CS, Zhang CL, Klaubert DH, Haugland RP (1999) A novel acidotrophic pH indicator and its potential application in labelling acidic organelles in live cells. Chem Biol 6 411–418 [DOI] [PubMed] [Google Scholar]
- Epimashko S, Meckel T, Fischer-Schliebs E, Lüttge U, Thiel G (2004) Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll leaf cell. Plant J 37 294–300 [DOI] [PubMed] [Google Scholar]
- Fleurat-Lessard P (1988) Structural and ultrastructural features of cortical cells in motor organs of sensitive plants. Biol Rev Camb Philos Soc 63 1–22 [Google Scholar]
- Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain JL, Martinoia E (1997) Increased expression of vacuolar aquaporin and H-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol 114 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurat-Lessard P, Michonneau P, Maeshima M, Drevon JJ, Serraj R (2005) The distribution of aquaporin subtypes (PIP1, PIP2 and gamma-TIP) is tissue dependent in soybean (Glycine max) root nodules. Ann Bot (Lond) 96 457–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flückiger R, De Caroli M, Piro G, Dalessandro G, Neuhaus J-M, Di Sansebastiano GP (2003) Vacuolar system distribution in Arabidopsis tissues, visualized using GFP fusion proteins. J Exp Bot 54 1577–1584 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Wittenbach VA, Giaquinta RT (1983) Paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation: III. Immunohistochemical localization of specific glycoproteins in the vacuole after depodding. Plant Physiol 72 586–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JS, Keller GA, Chrispeels MJ (1984) Localization of phytohemagglutinin in the embryonic axis of Phaseolus vulgaris with ultra-thin cryosections embedded in plastic after indirect immunolabeling. Planta 162 548–555 [DOI] [PubMed] [Google Scholar]
- Hall TC, Chandrasekharan MB, Li G (1999) Phaseolin: its past, properties, regulation, and future. In PR Shewry, R Casey, eds, Seed Proteins. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 209–240
- Hara-Nishimura I, Maeshima M (2000) Vacuolar processing enzymes and aquaporins. In DG Robinson, JC Rogers, eds, Vacuolar Compartments. Annual Plant Reviews, Vol 5. Sheffield Academic Press, Sheffield, UK, pp 20–42
- Haschke HP, Bremberger C, Lüttge U (1989) Transport proteins in plants with crassulacean metabolism: immunological characterization of ATPase subunits. In J Dainty, MI DeMichaelis, E Marré, E Rasi-Caldogno, eds, Plant Membrane Transport: The Current Position. Elsevier, Amsterdam, pp 149–154
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hinz G, Hillmer S, Bäumer M, Hohl I (1999) Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11 1509–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H, Hubbard L, Reizer J, Ludevid D, Herman EM, Chrisppeels MJ (1992) Vegetative and seed-specific forms of tonoplast intrinsic protein in the vacuolar membrane of Arabidopsis thaliana. Plant Physiol 99 561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh I, Hinz G, Jeong B-K, Robinson DG (1995) Protein storage vacuoles form de novo during pea cotyledon development. J Cell Sci 108 299–310 [DOI] [PubMed] [Google Scholar]
- Hohl I, Robinson DG, Chrispeels MJ, Hinz G (1996) Transport of vacuolar storage proteins is mediated by vesicles without a clathrin coat. J Cell Sci 109 2539–2550 [DOI] [PubMed] [Google Scholar]
- Jauh GY, Fischer AM, Grimes HD, Ryan CA, Rogers JC (1998) delta-Tonoplast intrinsic protein defines unique plant vacuole functions. Proc Natl Acad Sci USA 95 12995–12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh G-Y, Phillips T, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuole functions. Plant Cell 11 1867–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW, Rogers JC (2001) The protein storage vacuole: a unique compound organelle. J Cell Biol 155 991–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC (2000) Biogenesis of the protein storage crystalloid. J Cell Biol 150 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Höfte H, Chrispeels MJ (1989) An abundant, highly conserved tonoplast protein in seeds. Plant Physiol 91 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjemtrup S, Borksenious O, Raikhel NV, Chrispeels MJ (1995) Targeting and release of phytohemagglutinin from the roots of bean seedlings. Plant Physiol 109 603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr LA, Randell RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193 265–275 [PubMed] [Google Scholar]
- Ludevid D, Höfte H, Himmelblau E, Chrispeels MJ (1992) The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol 100 1633–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M (1992) Characterization of the major integral protein of vacuolar membrane. Plant Physiol 98 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F (1999) Plant vacuoles. Plant Cell 11 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu Y, Hattori M, Jauh GY, Rogers JC (2003) Alpha tonoplast intrinsic protein is specifically associated with vacuole membrane involved in an autophagic process. Plant Cell Physiol 44 795–802 [DOI] [PubMed] [Google Scholar]
- Müntz K, Shutov AD (2002) Legumins and their functions in plants. Trends Plant Sci 7 340–344 [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Paris N (2006) Plant vacuoles: from biogenesis to function. In J Samaj, F Baluska, D Menzel, eds, Plant Endocytosis, 1. Springer-Verlag, Berlin, pp 63–82
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis cells starts in the multivesicular bodies. Plant Cell 18 2567–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Stanley CM, Jones RL, Rogers JC (1996) Plant cells contain two functionally distinct vacuoles. Cell 85 563–572 [DOI] [PubMed] [Google Scholar]
- Park M, Kim SJ, Vitale A, Hwang I (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernollet JC (1978) Protein bodies of seeds: biochemistry, biosynthesis, and degradation. Phytochemistry 17 1473–1480 [Google Scholar]
- Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S, Robinson DG (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channles in Xenopus oocytes expressing red-cell CHIP28 protein. Science 256 385–387 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hinz G (1997) Vacuole biogenesis and protein transport to the plant vacuole: a comparison with the yeast vacuole and animal lysosome. Protoplasma 197 1–25 [Google Scholar]
- Robinson DG, Rogers JC (2000) Vacuolar Compartments. Annual Plant Reviews, Vol 5. Sheffield Academic Press, Sheffield, UK
- Sanmartin M, Ordonez A, Sohn EJ, Robert S, Sanchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Fragne N, Maeshima M, Fleurat-Lessard P, Drevon JJ (1998) A gamma-TIP cross-reacting protein is abundant in the cortex of soybean N2-fixing nodules. Planta 206 681–684 [Google Scholar]
- Staswick PE (1994) Storage proteins of vegetative plant tissue. Annu Rev Plant Physiol Plant Mol Biol 45 303–322 [Google Scholar]
- Steedman HF (1957) Polyester wax: a new ribboning embedding medium for histology. Nature 179 1345. [DOI] [PubMed] [Google Scholar]
- Suga S, Maeshima M (2004) Water channel activity of radish plasma membrane aquaporins heterologously expressed in yeast and their modification by site-directed mutagenesis. Plant Cell Physiol 45 823–830 [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Bethke PC, Jones RL (1998) Barley aleurone cells contain two types of vacuoles. Characterization of lytic organelles by use of fluorescent probes. Plant Cell 10 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann J, Neubohn B, Müntz K (2000) Different functions of vicilin and legumin are reflected in the histopattern of globulin mobilization during germination of vetch (Vicia sativa L.). Planta 211 1–12 [DOI] [PubMed] [Google Scholar]
- Tomos AD, Leigh RA, Koroleva OA (2000) Spatial and temporal variation in vacuolar contents. In DG Robinson, JC Rogers, eds, Vacuolar Compartments. Annual Plant Reviews, Vol 5. Sheffield Academic Press, Sheffield, UK, pp 174–198
- Vitale A, Hinz G (2005) Sorting of proteins to storage vacuoles: how many mechanisms? Trends Pharmacol Sci 10 316–323 [DOI] [PubMed] [Google Scholar]
- Wessel D, Flügge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138 141–143 [DOI] [PubMed] [Google Scholar]
- Wink M (1993) The plant vacuole—a multifunctional compartment. J Exp Bot (Suppl) 44 231–246 [Google Scholar]
- Zakharov A, Giersberg M, Hosein F, Melzer M, Müntz K, Saalbach I (2004) Seed-specific promoters direct gene expression in non-seed tissue. J Exp Bot 55 1463–1471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.