Abstract
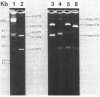
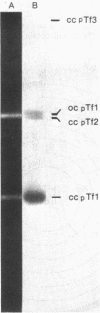
Three separate plasmids of 6, 7, 16, and greater than 23 kilobases were purified from a single clone of Thiobacillus ferrooxidans ATCC 33020 grown in the presence of uranium. The 6.7-kilobase plasmid (pTf1) was cloned separately into the HindIII or BamHI site of Escherichia coli plasmid pBR322. Restriction maps of the recombinant plasmids, termed pTf100 and pTf110, respectively, were constructed, creating potential cloning vehicles for exchanging genetic information between E. coli and T. ferrooxidans. Evidence from restriction enzyme analysis and Southern blot DNA-DNA hybridization indicates that the three native plasmids share little sequence homology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley C. L. Bacterial leaching. CRC Crit Rev Microbiol. 1978;6(3):207–26I. doi: 10.3109/10408417809090623. [DOI] [PubMed] [Google Scholar]
- Chopra I. Mechanism of plasmic-mediated resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1975 Jan;7(1):8–14. doi: 10.1128/aac.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg A. J., Holmes D. S. A note on the use of CsCl centrifugation to purify bacterial plasmids prepared by the rapid boiling method. Anal Biochem. 1982 Dec;127(2):434–434. doi: 10.1016/0003-2697(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Manning H. L. New medium for isolating iron-oxidizing and heterotrophic acidophilic bacteria from acid mine drainage. Appl Microbiol. 1975 Dec;30(6):1010–1016. doi: 10.1128/am.30.6.1010-1016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Dugan P. R., Tuovinen O. H. Plasmid DNA in acidophilic, chemolithotrophic thiobacilli. Can J Microbiol. 1981 Aug;27(8):850–853. doi: 10.1139/m81-133. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Studies on the growth of Thiobacillus ferrooxidans. I. Use of membrane filters and ferrous iron agar to determine viable numbers, and comparison with 14 CO 2 -fixation and iron oxidation as measures of growth. Arch Mikrobiol. 1973;88(4):285–298. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]