Abstract
We purified active hydrogenase from free-living Rhizobium japonicum by affinity chromatography. The uptake hydrogenase of R. japonicum has been treated previously as an oxygen-sensitive protein. In this purification, however, reducing agents were not added nor was there any attempt to exclude oxygen. In fact, the addition of sodium dithionite to aerobically purified protein resulted in the rapid loss of activity. Purified hydrogenase was more stable when stored under O2 than when stored under Ar. Sodium-chloride-washed hydrogen-oxidizing membranes were solubilized in Triton X-100 and deoxycholate and loaded onto a reactive red 120-agarose column. Purified hydrogenase elutes at 0.36 M NaCl, contains a nickel, and has a pH optimum of 6.0. There was 452-fold purification resulting in a specific activity of 76.9 mumol of H2 oxidized per min per mg of protein and a yield of 17%. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed subunits with estimated molecular weights of 65,000 and 33,000. Hydrogenase prepared in this manner was used to raise and affinity purify antibodies against both subunits.
Full text
PDF


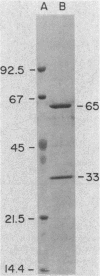
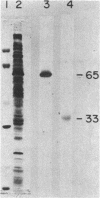
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E. The physical and catalytic properties of hydrogenase II of Clostridium pasteurianum. A comparison with hydrogenase I. J Biol Chem. 1984 Jun 10;259(11):7045–7055. [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Arp D. J. Rhizobium japonicum hydrogenase: purification to homogeneity from soybean nodules, and molecular characterization. Arch Biochem Biophys. 1985 Mar;237(2):504–512. doi: 10.1016/0003-9861(85)90303-0. [DOI] [PubMed] [Google Scholar]
- Ballantine S. P., Boxer D. H. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):454–459. doi: 10.1128/jb.163.2.454-459.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Spectral evidence for a component involved in hydrogen metabolism of soybean nodule bacteroids. Plant Physiol. 1982 Dec;70(6):1667–1672. doi: 10.1104/pp.70.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Schneider K., Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982 Oct;152(1):42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Lambert G. R., Hanus F. J., Evans H. J. Further evidence that two unique subunits are essential for expression of hydrogenase activity in Rhizobium japonicum. J Bacteriol. 1985 Oct;164(1):187–191. doi: 10.1128/jb.164.1.187-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Xu L. S., Hanus F. J., Evans H. J. Some properties of the nickel-containing hydrogenase of chemolithotrophically grown Rhizobium japonicum. J Bacteriol. 1984 Sep;159(3):850–856. doi: 10.1128/jb.159.3.850-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Czechowski M. H., Krüger H. J., DerVartanian D. V., Peck H. D., Jr, LeGall J. Desulfovibrio vulgaris hydrogenase: a nonheme iron enzyme lacking nickel that exhibits anomalous EPR and Mössbauer spectra. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3728–3732. doi: 10.1073/pnas.81.12.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger H. J., Huynh B. H., Ljungdahl P. O., Xavier A. V., Der Vartanian D. V., Moura I., Peck H. D., Jr, Teixeira M., Moura J. J., LeGall J. Evidence for nickel and a three-iron center in the hydrogenase of Desulfovibrio desulfuricans. J Biol Chem. 1982 Dec 25;257(24):14620–14623. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- Merberg D., O'Hara E. B., Maier R. J. Regulation of hydrogenase in Rhizobium japonicum: analysis of mutants altered in regulation by carbon substrates and oxygen. J Bacteriol. 1983 Dec;156(3):1236–1242. doi: 10.1128/jb.156.3.1236-1242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeremans M., Daneels G., De Mey J. Sensitive colloidal metal (gold or silver) staining of protein blots on nitrocellulose membranes. Anal Biochem. 1985 Mar;145(2):315–321. doi: 10.1016/0003-2697(85)90368-9. [DOI] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Expression of cytochrome o in hydrogen uptake constitutive mutants of Rhizobium japonicum. J Bacteriol. 1985 Feb;161(2):507–514. doi: 10.1128/jb.161.2.507-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Role of ubiquinone in hydrogen-dependent electron transport in Rhizobium japonicum. J Bacteriol. 1985 Feb;161(2):775–777. doi: 10.1128/jb.161.2.775-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Rieder R., Cammack R., Hall D. O. Purification and properties of the soluble hydrogenase from Desulfovibrio desulfuricans (strain Norway 4). Eur J Biochem. 1984 Dec 17;145(3):637–643. doi: 10.1111/j.1432-1033.1984.tb08604.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Argüeso T., Emerich D. W., Evans H. J. Hydrogenase system in legume nodules: a mechanism of providing nitrogenase with energy and protection from oxygen damage. Biochem Biophys Res Commun. 1979 Jan 30;86(2):259–264. doi: 10.1016/0006-291x(79)90860-x. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schneider K., Pinkwart M., Jochim K. Purification of hydrogenases by affinity chromatography on Procion Red-agarose. Biochem J. 1983 Aug 1;213(2):391–398. doi: 10.1042/bj2130391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell C. P., Kuhlenschmidt T. B., Hoppe C. A. A fluorescamine assay for submicrogram quantities of protein in the presence of Triton X-100. Anal Biochem. 1978 Apr;85(2):572–580. doi: 10.1016/0003-2697(78)90256-7. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Böcher R., Knecht J., Kröger A. Hydrogenase from Vibrio succinogenes, a nickel protein. FEBS Lett. 1982 Aug 23;145(2):230–234. doi: 10.1016/0014-5793(82)80173-7. [DOI] [PubMed] [Google Scholar]
- van der Zwaan J. W., Albracht S. P., Fontijn R. D., Slater E. C. Monovalent nickel in hydrogenase from Chromatium vinosum. Light sensitivity and evidence for direct interaction with hydrogen. FEBS Lett. 1985 Jan 7;179(2):271–277. doi: 10.1016/0014-5793(85)80533-0. [DOI] [PubMed] [Google Scholar]