Abstract
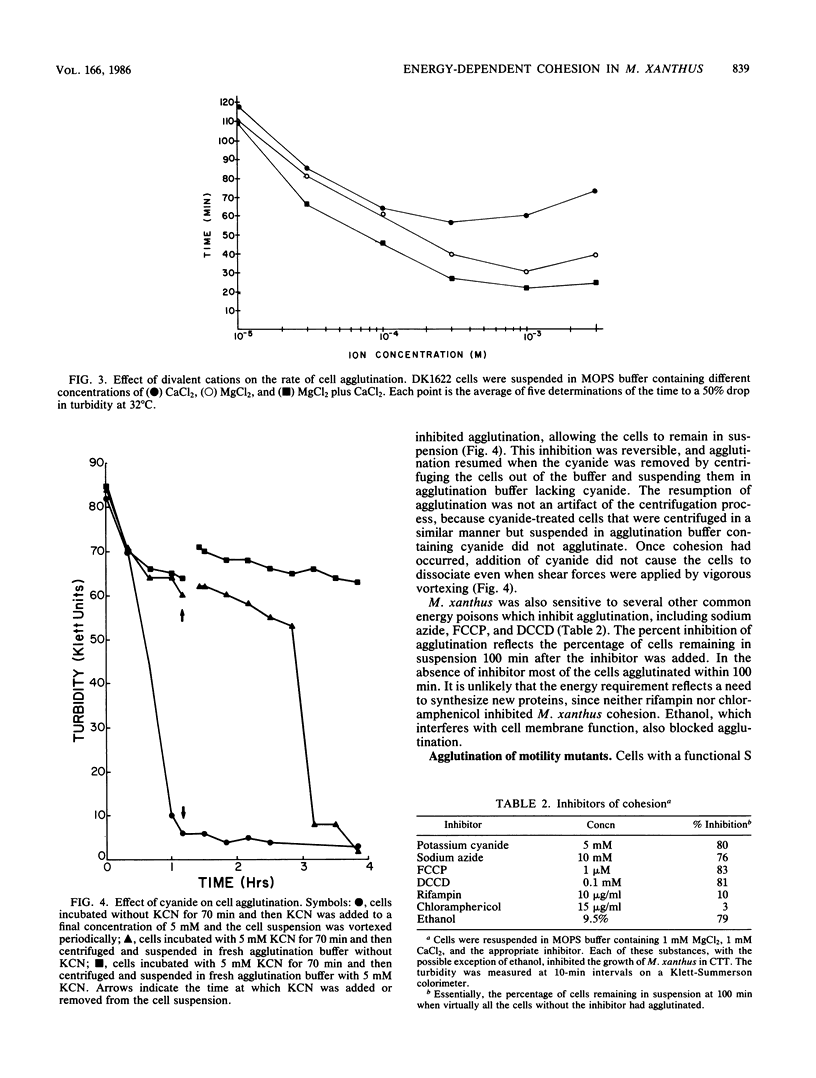
An agglutination assay was used to study cell cohesion in the myxobacterium Myxococcus xanthus. Vegetative cells agglutinated in the presence of the divalent cations Mg2+ and Ca2+. Agglutination was blocked by energy poisons that inhibit electron transport, uncouple oxidative phosphorylation, or inhibit the membrane-bound ATPase. However, energy was not required for the maintenance of cells in the multicellular aggregate. Cyanide, a strong inhibitor of agglutination, did not cause cells to dissociate from the aggregate even when shear forces were applied. While gliding motility was not necessary for agglutination, some gliding mutants exhibited aberrant agglutination that was generally correlated with cell behavior. Cells with an intact social motility system were cohesive and glided in large multicellular swarms. Cells with a mutation in their social motility system were 5- to 10-fold less cohesive and tended to glide as single cells. One group of social motility mutants, known as Dsp, did not agglutinate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P. Studies on gliding motility in Myxococcus xanthus. Arch Microbiol. 1974;99(3):271–280. doi: 10.1007/BF00696242. [DOI] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Gilmore D. F., White D. Energy-dependent cell cohesion in myxobacteria. J Bacteriol. 1985 Jan;161(1):113–117. doi: 10.1128/jb.161.1.113-117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae T. H., Dobson W. J., McCurdy H. D. Fimbriation in gliding bacteria. Can J Microbiol. 1977 Aug;23(8):1096–1108. doi: 10.1139/m77-165. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986 Jun;166(3):842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



