Abstract
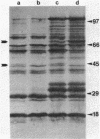
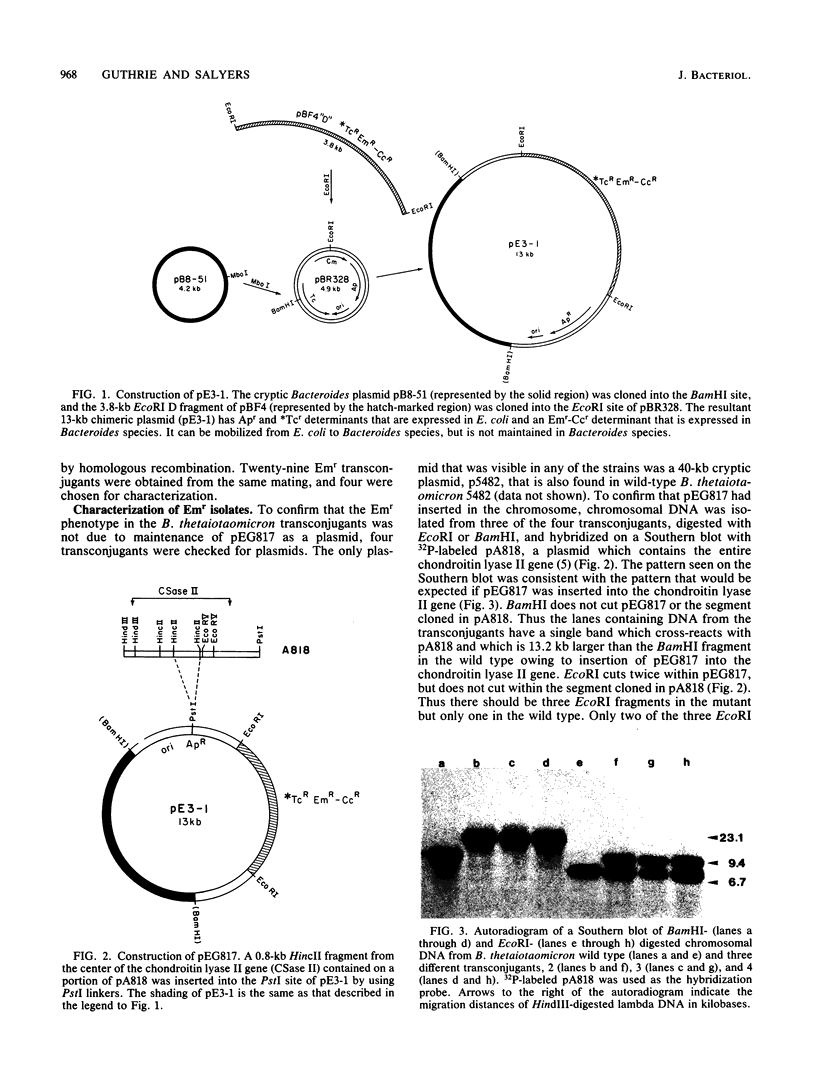
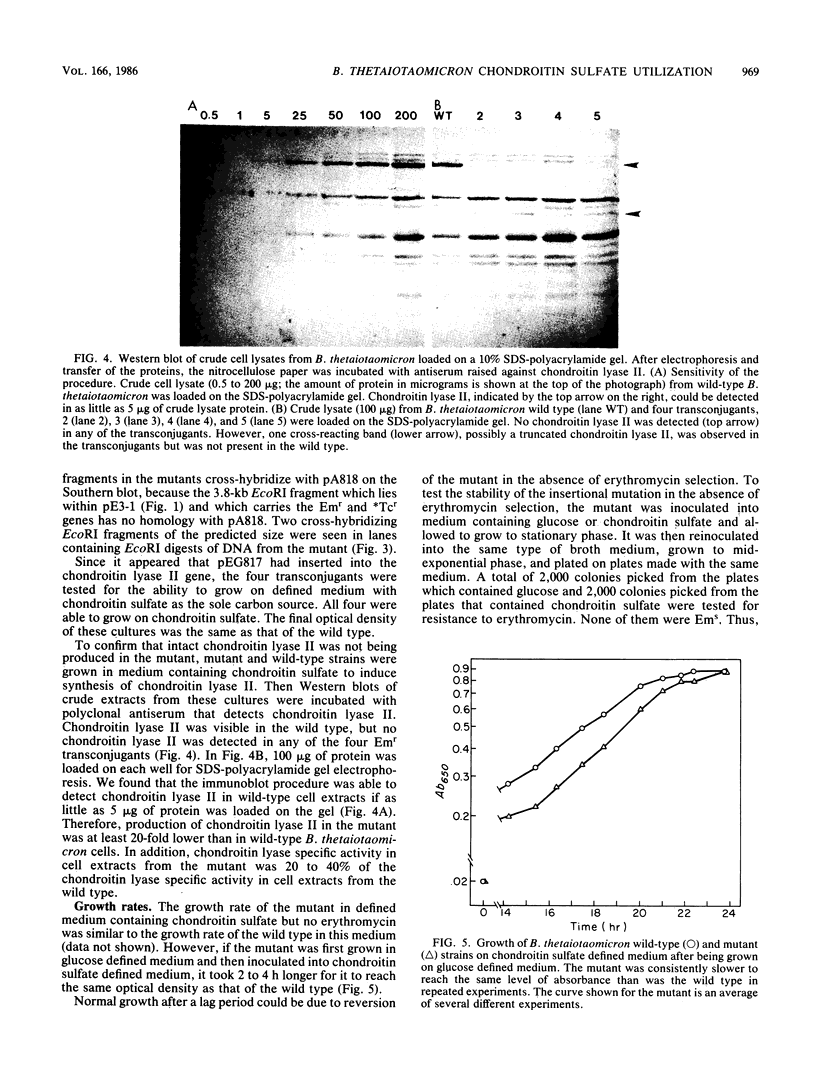
Bacteroides thetaiotaomicron produces two inducible chondroitin lyases (I and II) when it is grown on chondroitin sulfate. Both enzymes have very similar biochemical properties. To determine whether both enzymes are required for growth on chondroitin sulfate, we constructed a Bacteroides suicide vector, pE3-1, and used it to create an insertional mutation that interrupts the chondroitin lyase II gene of Bacteroides thetaiotaomicron. pE3-1 contains a 4.4-kilobase cryptic B. eggerthii plasmid (pB8-51), the Escherichia coli cloning vector pBR328, and the EcoRI D fragment from the conjugative B. fragilis plasmid pBF4. A 0.8-kilobase fragment from the center of the B. thetaiotaomicron chondroitin lyase II gene was inserted in pE3-1 to create pEG817. Although, pEG817 is stably maintained in E. coli and can be mobilized into B. thetaiotaomicron by the IncP plasmid R751, pEG817 is not maintained as a plasmid in Bacteroides spp. When pEG817 was mobilized into B. thetaiotaomicron, with selection for a drug marker on pEG817, transconjugants were obtained which had pEG817 inserted into the chondroitin lyase II gene. Western blot analysis was used to confirm that intact chondroitin lyase II is not produced in the mutant. The mutant was able to utilize chondroitin sulfate as a sole source of carbon, although no active chondroitin lyase II was produced. Thus chondroitin lyase I alone appears to be sufficient for growth on chondroitin sulfate. The mutant also had some minor changes in its outer membrane protein profile. However, there was no evidence that any of the major chondroitin sulfate-associated polypeptides in the outer membrane were affected by the insertion in the chondroitin lyase II gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Jr, Hasegawa P., Davis C. E. Expression in Escherichia coli of cryptic tetracycline resistance genes from bacteroides R plasmids. Plasmid. 1984 May;11(3):248–252. doi: 10.1016/0147-619x(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Guthrie E. P., Shoemaker N. B., Salyers A. A. Cloning and expression in Escherichia coli of a gene coding for a chondroitin lyase from Bacteroides thetaiotaomicron. J Bacteriol. 1985 Nov;164(2):510–515. doi: 10.1128/jb.164.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Linz J., Braun D. M., Salyers A. A. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. doi: 10.1128/jb.163.3.1080-1086.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipeski L., Guthrie E. P., O'Brien M., Kotarski S. F., Salyers A. A. Comparison of proteins involved in chondroitin sulfate utilization by three colonic Bacteroides species. Appl Environ Microbiol. 1986 May;51(5):978–984. doi: 10.1128/aem.51.5.978-984.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin D. E., Voss E. W., Jr Anti-lysergyl antibody: measurement of binding parameters in IgG fractions. Immunochemistry. 1974 Jun;11(6):285–293. doi: 10.1016/0019-2791(74)90364-4. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. Polyethylene glycol-facilitated transformation of Bacteroides fragilis with plasmid DNA. J Bacteriol. 1985 Oct;164(1):466–469. doi: 10.1128/jb.164.1.466-469.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]