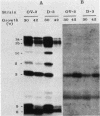
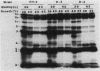
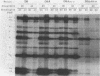
Abstract
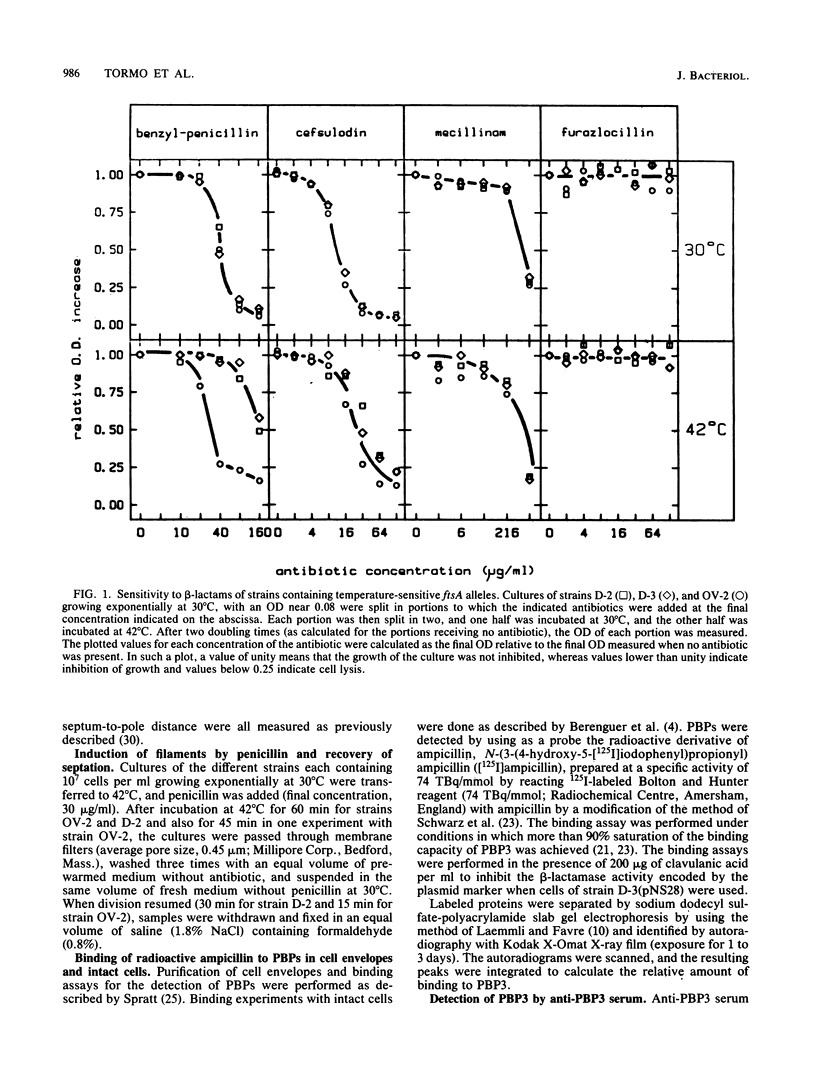
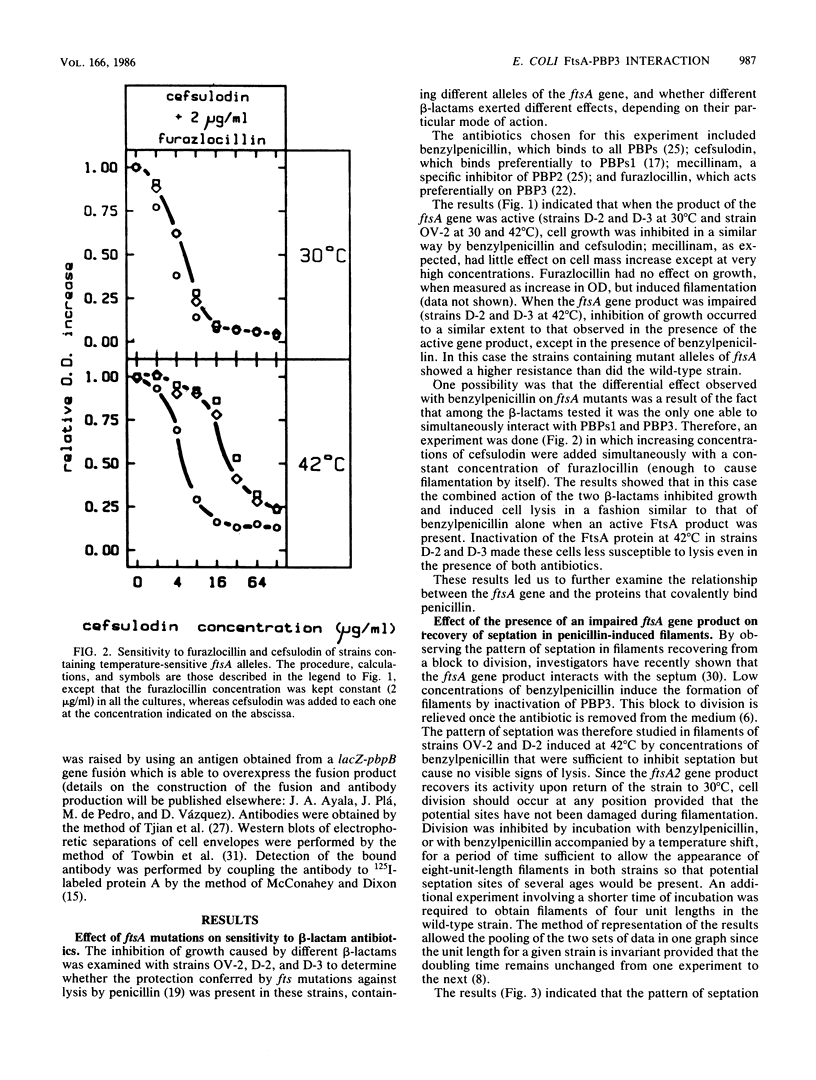
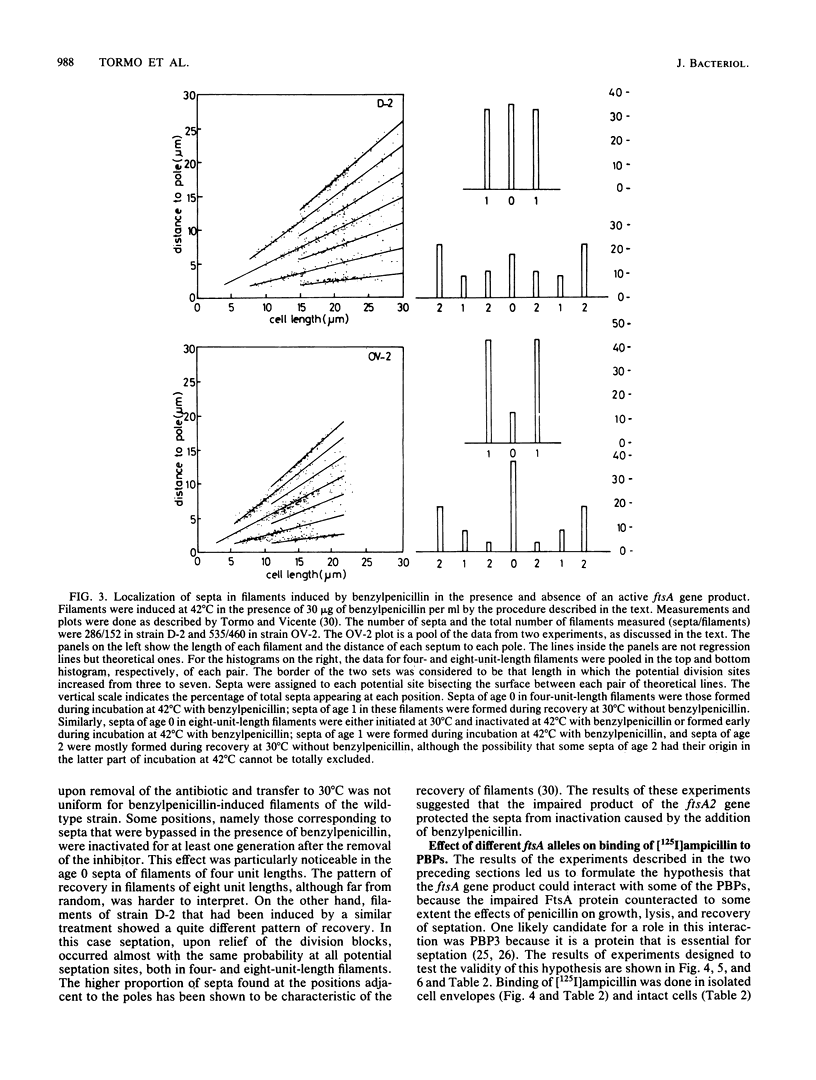
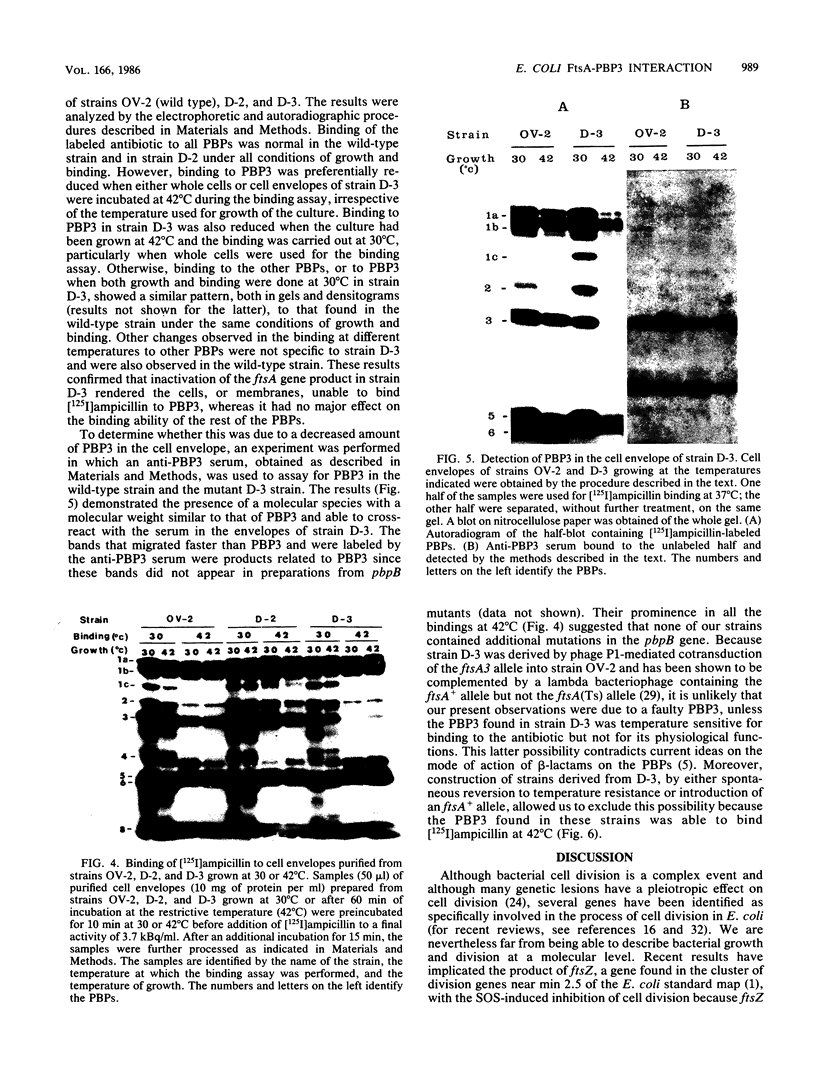
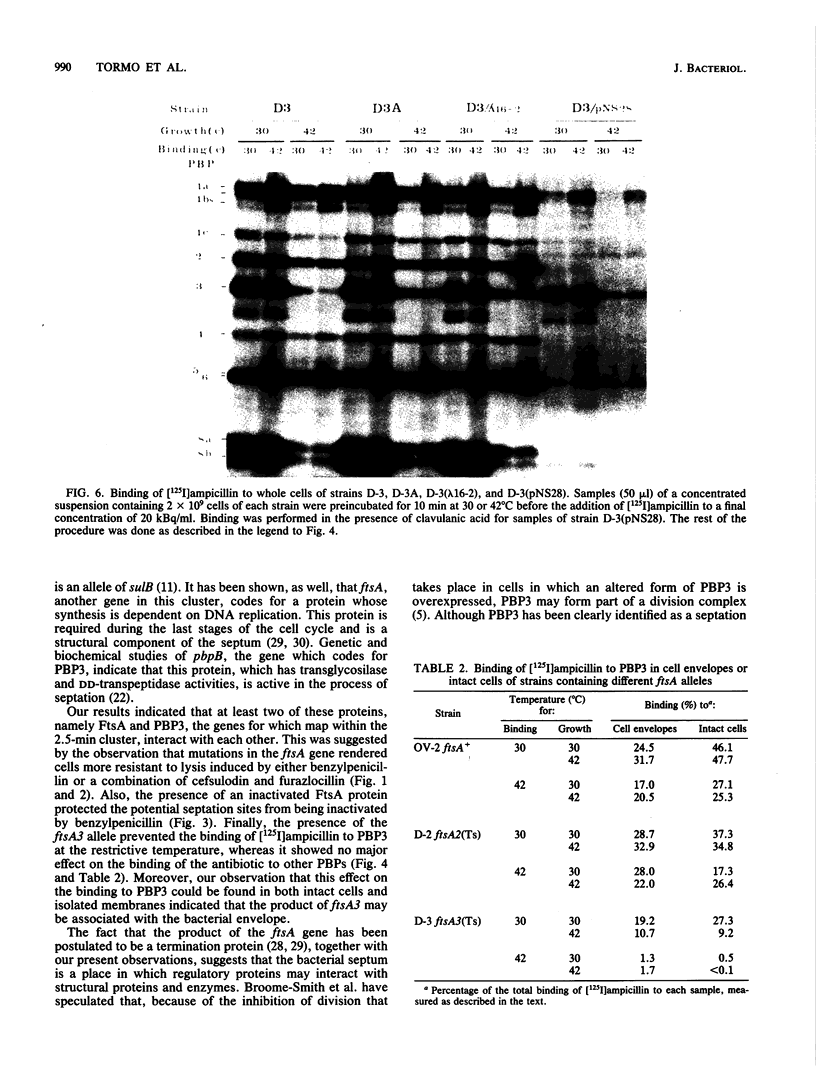
Mutations in the ftsA gene of Escherichia coli conferred a higher resistance to lysis induced by penicillin or by a combination of cefsulodin and furazlocillin. The ftsA2 allele codes for an FtsA protein which is inactive at 42 degrees C but is able to regain its activity once it is transferred back to 30 degrees C; ftsA2 filaments formed at 42 degrees C in the presence of penicillin divided once the penicillin was removed and the temperature was lowered to 30 degrees C. Potential septation sites in the filaments of wild-type cells treated in the same way remained inactive. The binding of a radioactively labeled derivative of ampicillin to penicillin-binding protein 3 (PBP3) was significantly decreased in strain D-3, containing the mutant allele ftsA3, when the binding assay was performed at the restrictive temperature. A molecular species able to cross-react with an anti-PBP3 serum was nevertheless found to be present in the envelope of D-3 cells. These observations suggested that the FtsA protein, a protein with a structural and regulatory role in septation, and PBP3, a protein enzymatically active in the synthesis of murein for septation, interact with each other.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J Bacteriol. 1985 Aug;163(2):615–622. doi: 10.1128/jb.163.2.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Hatfull G. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell division gene ftsQ. J Bacteriol. 1980 Oct;144(1):435–437. doi: 10.1128/jb.144.1.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer J., De Pedro M. A., Vázquez D. V. Interaction of nocardicin A with the penicillin-binding proteins of Escherichia coli in intact cells and in purified cell envelopes. Eur J Biochem. 1982 Aug;126(1):155–159. doi: 10.1111/j.1432-1033.1982.tb06760.x. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Hedge P. J., Spratt B. G. Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site-directed mutagenesis. EMBO J. 1985 Jan;4(1):231–235. doi: 10.1002/j.1460-2075.1985.tb02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Lutkenhaus J. F., Salmond G. P., Martinez-Salas E., Vincente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979 Nov;140(2):388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Fletcher G., Irwin C. A., Henson J. M., Fillingim C., Malone M. M., Walker J. R. Identification of the Escherichia coli cell division gene sep and organization of the cell division-cell envelope genes in the sep-mur-ftsA-envA cluster as determined with specialized transducing lambda bacteriophages. J Bacteriol. 1978 Jan;133(1):91–100. doi: 10.1128/jb.133.1.91-100.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Donachie W. D. Identification of the ftsA gene product. J Bacteriol. 1979 Mar;137(3):1088–1094. doi: 10.1128/jb.137.3.1088-1094.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Wolf-Watz H., Donachie W. D. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J Bacteriol. 1980 May;142(2):615–620. doi: 10.1128/jb.142.2.615-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Salas E., Vicente M. Amber mutation affecting the length of Escherichia coli cells. J Bacteriol. 1980 Nov;144(2):532–541. doi: 10.1128/jb.144.2.532-541.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H. Bacterial growth and division: genes, structures, forces, and clocks. Microbiol Rev. 1982 Sep;46(3):341–375. doi: 10.1128/mr.46.3.341-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S., Hagenzieker J. G. Constriction and septation during cell division in caulobacters. Can J Microbiol. 1981 Jul;27(7):704–719. doi: 10.1139/m81-109. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Kenan D. J., Hatfull G. F., Sullivan N. F., Spiegelberg R., Donachie W. D. DNA sequence and transcriptional organization of essential cell division genes ftsQ and ftsA of Escherichia coli: evidence for overlapping transcriptional units. J Bacteriol. 1984 Nov;160(2):546–555. doi: 10.1128/jb.160.2.546-555.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Ayala J. A., de la Rosa E. J., de Pedro M. A., Arán V., Berenguer J., Vázquez D. Binding of 125I-labeled beta-lactam antibiotics to the penicillin binding proteins of Escherichia coli. J Antibiot (Tokyo) 1984 Apr;37(4):389–393. doi: 10.7164/antibiotics.37.389. [DOI] [PubMed] [Google Scholar]
- Schmidt L. S., Botta G., Park J. T. Effects of furazlocillin, a beta-lactam antibiotic which binds selectively to penicillin-binding protein 3, on Escherichia coli mutants deficient in other penicillin-binding proteins. J Bacteriol. 1981 Jan;145(1):632–637. doi: 10.1128/jb.145.1.632-637.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Stinchcomb D., Losick R. Antibody directed against Bacillus subtilis rho factor purified by sodium dodecyl sulfate slab gel electrophoresis. Effect on transcription by RNA polymerase in crude extracts of vegetative and sporulating cells. J Biol Chem. 1975 Nov 25;250(22):8824–8828. [PubMed] [Google Scholar]
- Tormo A., Fernández-Cabrera C., Vicente M. The ftsA gene product: a possible connection between DNA replication and septation in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):239–244. doi: 10.1099/00221287-131-2-239. [DOI] [PubMed] [Google Scholar]
- Tormo A., Martínez-Salas E., Vicente M. Involvement of the ftsA gene product in late stages of the Escherichia coli cell cycle. J Bacteriol. 1980 Feb;141(2):806–813. doi: 10.1128/jb.141.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes F. B., Olijhoek T. J., Schwarz U., Nanninga N. Labeling pattern of major penicillin-binding proteins of Escherichia coli during the division cycle. J Bacteriol. 1983 Mar;153(3):1287–1293. doi: 10.1128/jb.153.3.1287-1293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]