Abstract
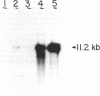
The stability of two ColE1-related plasmids (pRSF2124 and pMB9) was examined in strains of Escherichia coli multiply deficient in exonucleases I (sbcB), III (xthA), or V (recB recC). Any combination of exonuclease I, III, and V deficiency resulted in dramatically decreased stability of both pRSF2124 and pMB9. Inactivation of the RecF pathway by introducing either recF or recJ mutations to the recB recC subcB background resulted in nearly wild-type levels of stability for both plasmids. In contrast, the introduction of uvrD3 uvr-257, uvrE100, or recL152 into the recB21 recC22 sbcB15 strain did not affect plasmid stability. Furthermore, the amount of plasmid DNA recovered from pRSF2124 or pMB9 transformants of a xthA1 sbcB15 strain was strikingly reduced relative to that of a wild-type control. Taken together, these results suggest that some aspect of DNA repair is required for stable maintenance of ColE1-related plasmids in E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R., Vapnek D. In vivo transcription of R-plasmid deoxyribonucleic acid in Escherichia coli strains with altered antibiotic resistance levels and/or conjugal proficiency. J Bacteriol. 1976 Mar;125(3):1148–1155. doi: 10.1128/jb.125.3.1148-1155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Inselburg J. ColE1 plasmid mutants affecting growth of an Escherichia coli recB recC sbcB mutant. J Bacteriol. 1978 Jan;133(1):433–436. doi: 10.1128/jb.133.1.433-436.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meacock P. A., Cohen S. N. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980 Jun;20(2):529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., DE MARGERIE H. Production of colicine by single bacteria. Nature. 1959 Aug 1;184:337–339. doi: 10.1038/184337a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA added to cell extracts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1050–1054. doi: 10.1073/pnas.72.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Alton N. K., Bassett C. L., Kushner S. R. Amplification in Escherichia coli of enzymes involved in genetic recombination: construction of hybrid ColE1 plasmids carrying the structural gene for exonuclease I. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3492–3496. doi: 10.1073/pnas.73.10.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston A., Brown M. G., Perkins H. R., Saunders J. R., Humphreys G. O. Transformation of Escherichia coli with plasmid deoxyribonucleic acid: calcium-induced binding of deoxyribonucleic acid to whole cells and to isolated membrane fractions. J Bacteriol. 1981 Feb;145(2):780–787. doi: 10.1128/jb.145.2.780-787.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Maples V. F., Kushner S. R. Recombinant levels of Escherichia coli K-12 mutants deficient in various replication, recombination, or repair genes. J Bacteriol. 1978 Jun;134(3):958–966. doi: 10.1128/jb.134.3.958-966.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]