Abstract
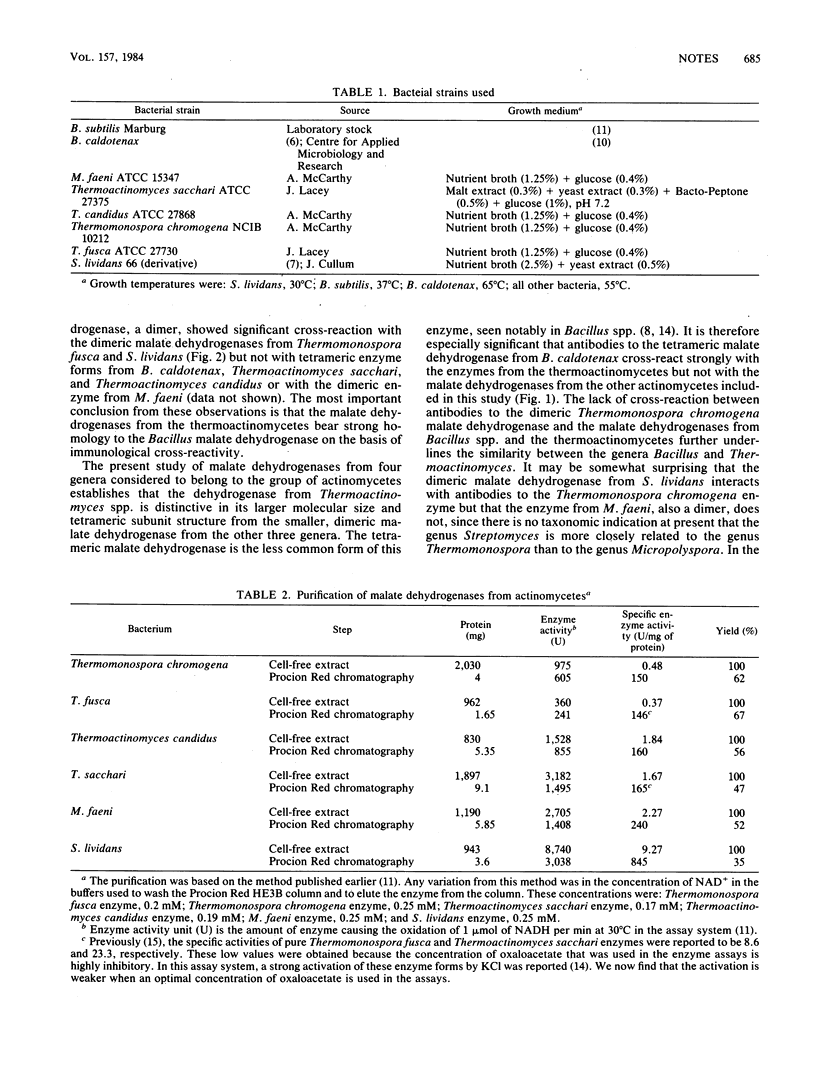
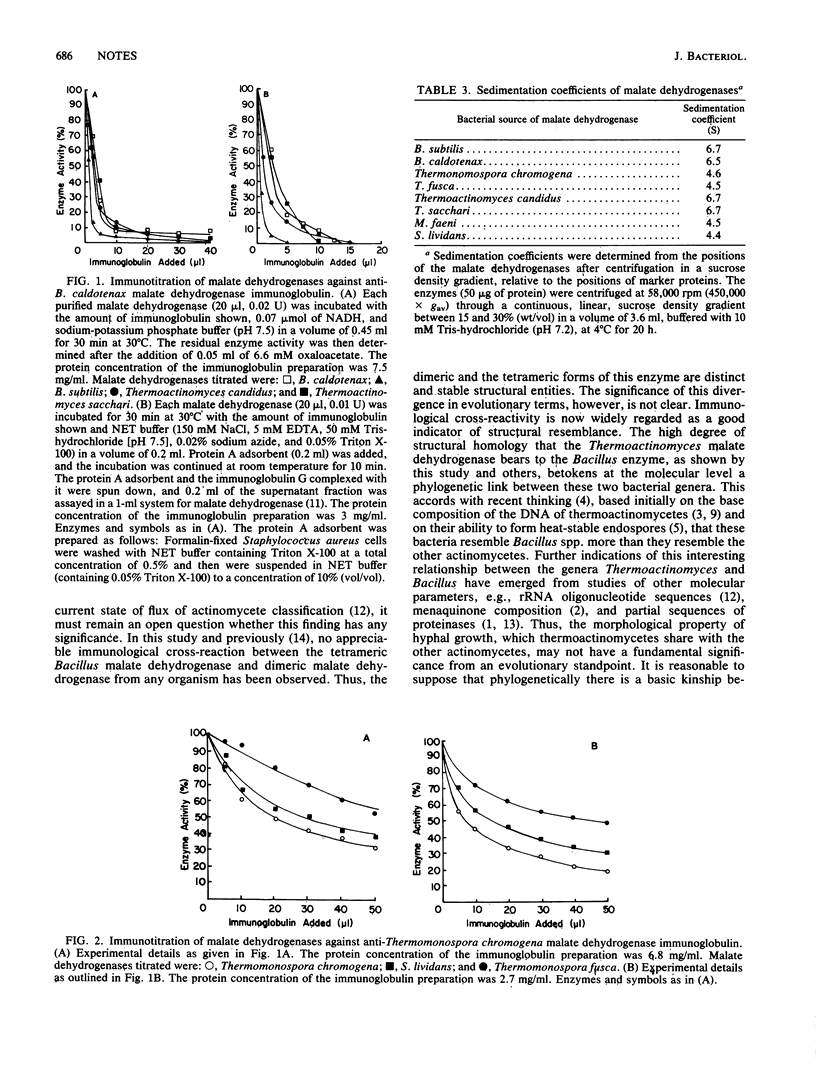
Malate dehydrogenases from bacteria belonging to the genus Thermoactinomyces are tetrameric, like those from Bacillus spp., and exhibit a high degree of structural homology to Bacillus malate dehydrogenase as judged by immunological cross-reactivity. Malate dehydrogenases from other actinomycetes are dimers and do not cross-react with antibodies to Bacillus malate dehydrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudys M., Kostka V., Grüner K., Hausdorf G., Höhne W. E. Amino acid sequence of the small cyanogen bromide peptide of thermitase, a thermostable serine proteinase from Thermoactinomyces vulgaris. Relation to the subtilisins. Int J Pept Protein Res. 1982 Jan;19(1):32–39. doi: 10.1111/j.1399-3011.1982.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Cross T., Walker P. D., Gould G. W. Thermophilic actinomycetes producing resistant endospores. Nature. 1968 Oct 26;220(5165):352–354. doi: 10.1038/220352a0. [DOI] [PubMed] [Google Scholar]
- Heinen U. J., Heinen W. Characteristics and properties of a caldo-active bacterium producing extracellular enzymes and two related strains. Arch Mikrobiol. 1972;82(1):1–23. doi: 10.1007/BF00424925. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey W. H., Kitto G. B., Everse J., Kaplan N. Malate dehydrogenases. I. A survey of molecular size measured by gel filtration. Biochemistry. 1967 Feb;6(2):603–610. doi: 10.1021/bi00854a031. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Chestukhina G. G., Rudenskaya G. N., Epremyan A. S., Osterman A. L., Khodova O. M., Belyanova L. P. A new subfamily of microbial serine proteinase? Structural similarities of Bacillus thuringiensis and Thermoactinomyces vulgaris extracellular serine proteinases. Biochem Biophys Res Commun. 1981 Jun;100(4):1680–1687. doi: 10.1016/0006-291x(81)90712-9. [DOI] [PubMed] [Google Scholar]
- Sundaram T. K., Wright I. P., Wilkinson A. E. Malate dehydrogenase from thermophilic and mesophilic bacteria. Molecular size, subunit structure, amino acid composition, immunochemical homology, and catalytic activity. Biochemistry. 1980 May 13;19(10):2017–2022. doi: 10.1021/bi00551a002. [DOI] [PubMed] [Google Scholar]
- Wright I. P., Sundaram T. K. Simple efficient methods for the isolation of malate dehydrogenase from thermophilic and mesophilic bacteria. Biochem J. 1979 Feb 1;177(2):441–448. doi: 10.1042/bj1770441. [DOI] [PMC free article] [PubMed] [Google Scholar]


