Abstract
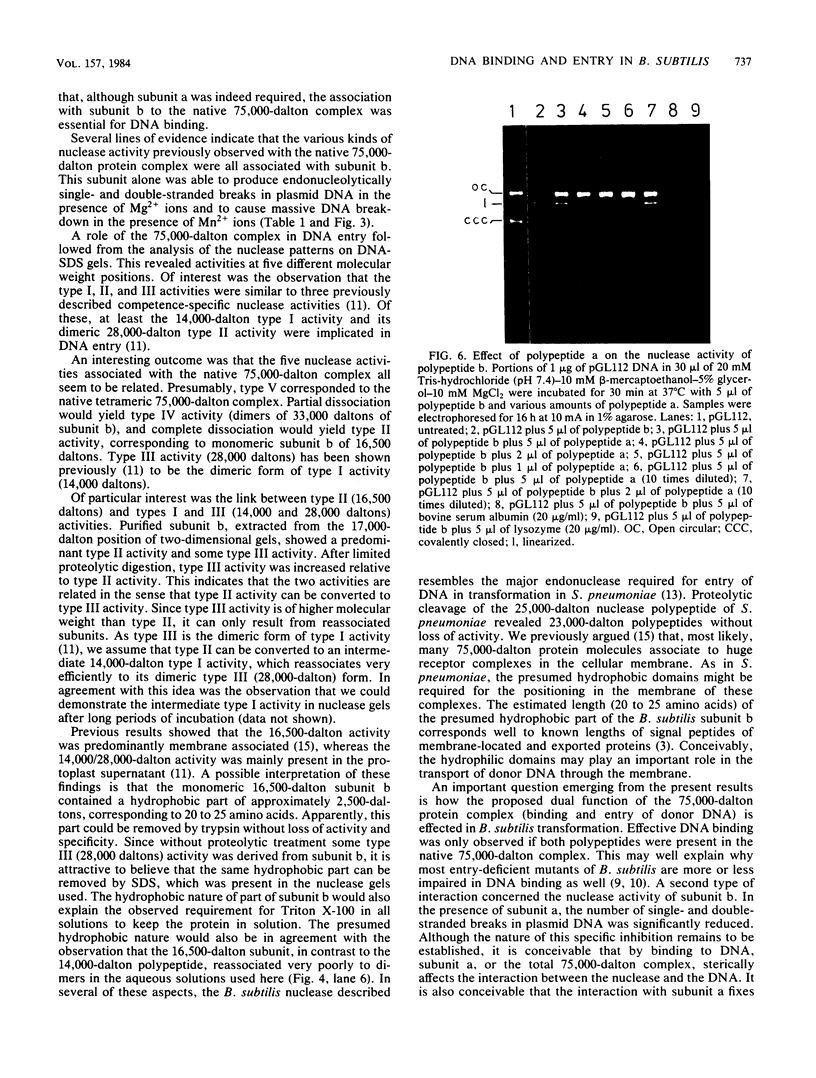
A 75,000-dalton protein complex involved in DNA binding during transformation was purified from membranes of competent Bacillus subtilis cells. Previous results (Smith et al., J. Bacteriol. 156:101-108, 1983) showed that the complex contained two polypeptides, polypeptide a (molecular weight, 18,000; isoelectric point, 5.0) and polypeptide b (molecular weight, 17,000; isoelectric point, 4.7) in approximately equal amounts. In the present experiments the two polypeptides were extracted from two-dimensional gels and studied separately and in combination with respect to DNA binding and nuclease activities. For DNA binding the interaction of both polypeptides was required. DNA binding occurred efficiently in the presence of EDTA. Nuclease activity was restricted to polypeptide b. The nucleolytic properties of b were identical to those of the native 75,000-dalton complex. Polypeptide a affected b by reducing its nuclease activity. Analysis of the nuclease subunit b on DNA-containing polyacrylamide gels revealed nuclease activities at four different molecular weight positions. These activities were identical to the major competence-specific nuclease activities which were previously implicated in the entry of donor DNA during transformation (Mulder and Venema, J. Bacteriol. 152:166-174, 1982). These results indicate that the 75,000-dalton protein complex is composed of two different competence-specific polypeptides involved in both binding and entry of donor DNA. The possible roles of the two polypeptides in the transformation of B. subtilis are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bron S., Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972 May;15(1):1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Isolation and partial characterization of Bacillus subtilis mutants impaired in DNA entry. J Bacteriol. 1982 Apr;150(1):260–268. doi: 10.1128/jb.150.1.260-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Transformation-deficient mutants of Bacillus subtilis impaired in competence-specific nuclease activities. J Bacteriol. 1982 Oct;152(1):166–174. doi: 10.1128/jb.152.1.166-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Complex structure of the membrane nuclease of Streptococcus pneumoniae revealed by two-dimensional electrophoresis. J Mol Biol. 1980 Aug 5;141(2):133–146. doi: 10.1016/0022-2836(80)90381-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Smith H., Wiersma K., Bron S., Venema G. Transformation in Bacillus subtilis: purification and partial characterization of a membrane-bound DNA-binding protein. J Bacteriol. 1983 Oct;156(1):101–108. doi: 10.1128/jb.156.1.101-108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., de Vos W., Bron S. Transformation in Bacillus subtilis: properties of DNA-binding-deficient mutants. J Bacteriol. 1983 Jan;153(1):12–20. doi: 10.1128/jb.153.1.12-20.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema G. Bacterial transformation. Adv Microb Physiol. 1979;19:245–331. doi: 10.1016/s0065-2911(08)60200-3. [DOI] [PubMed] [Google Scholar]