Abstract
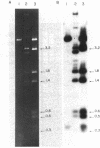
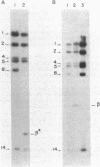
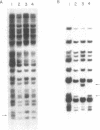
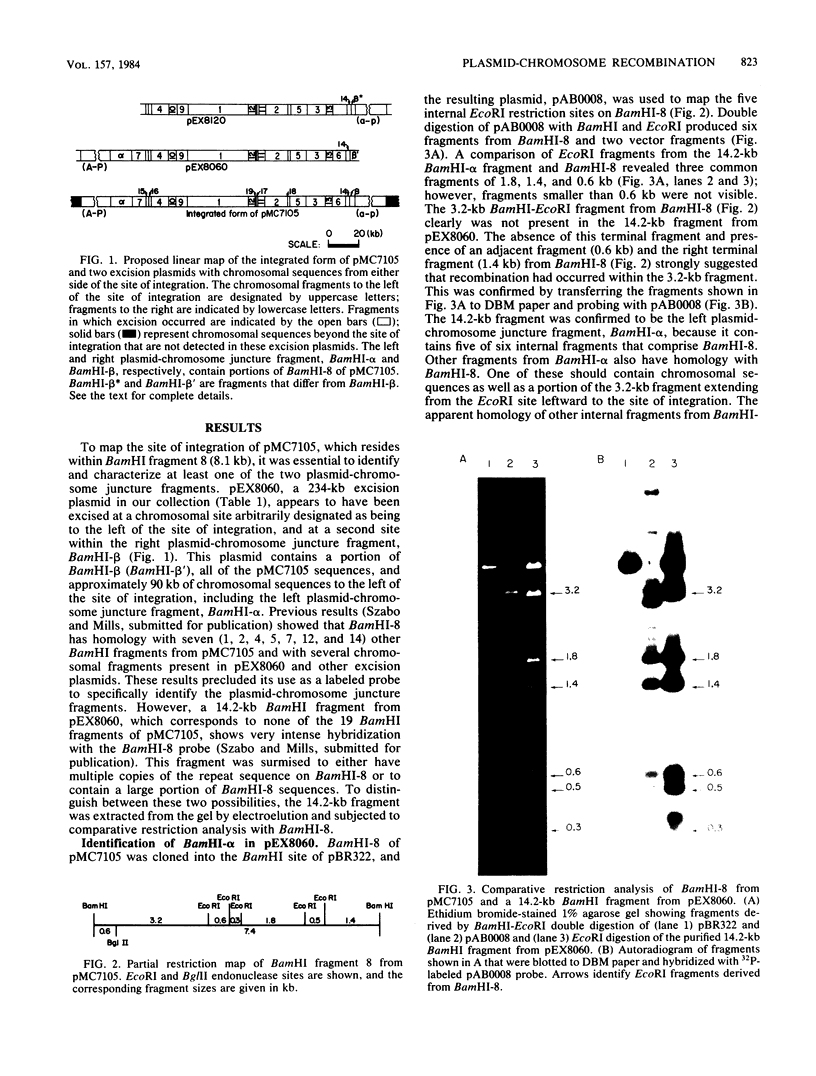
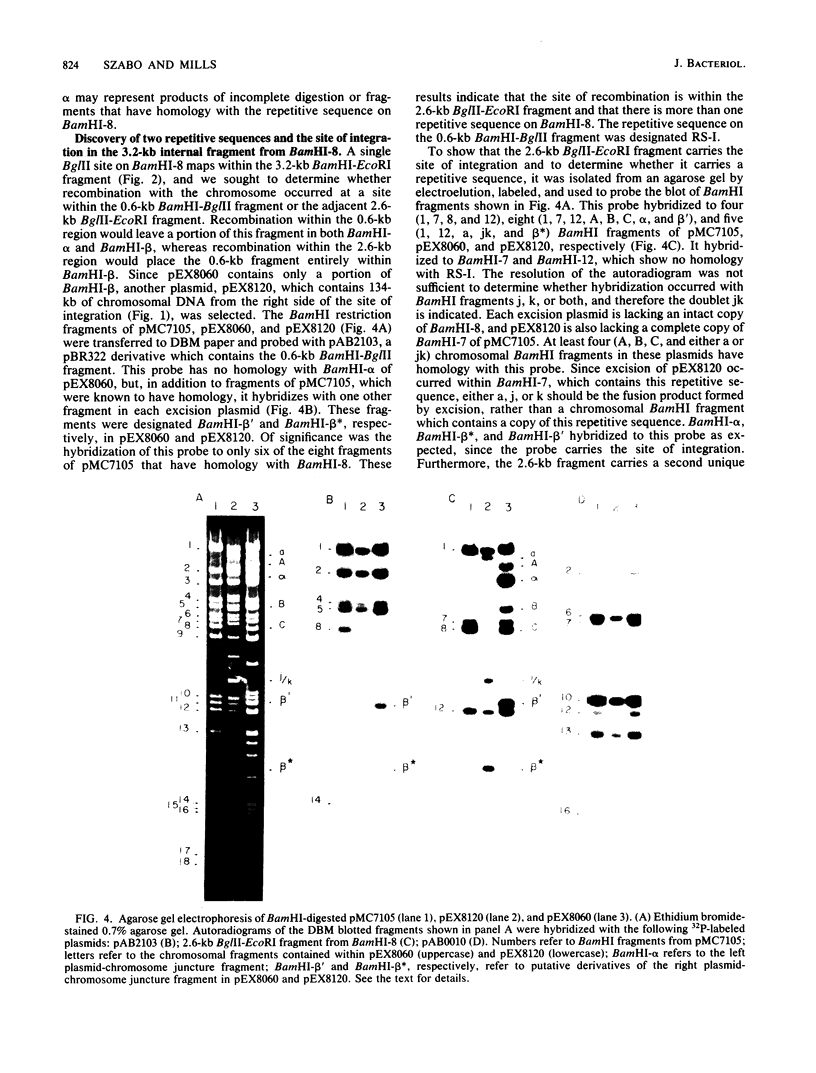
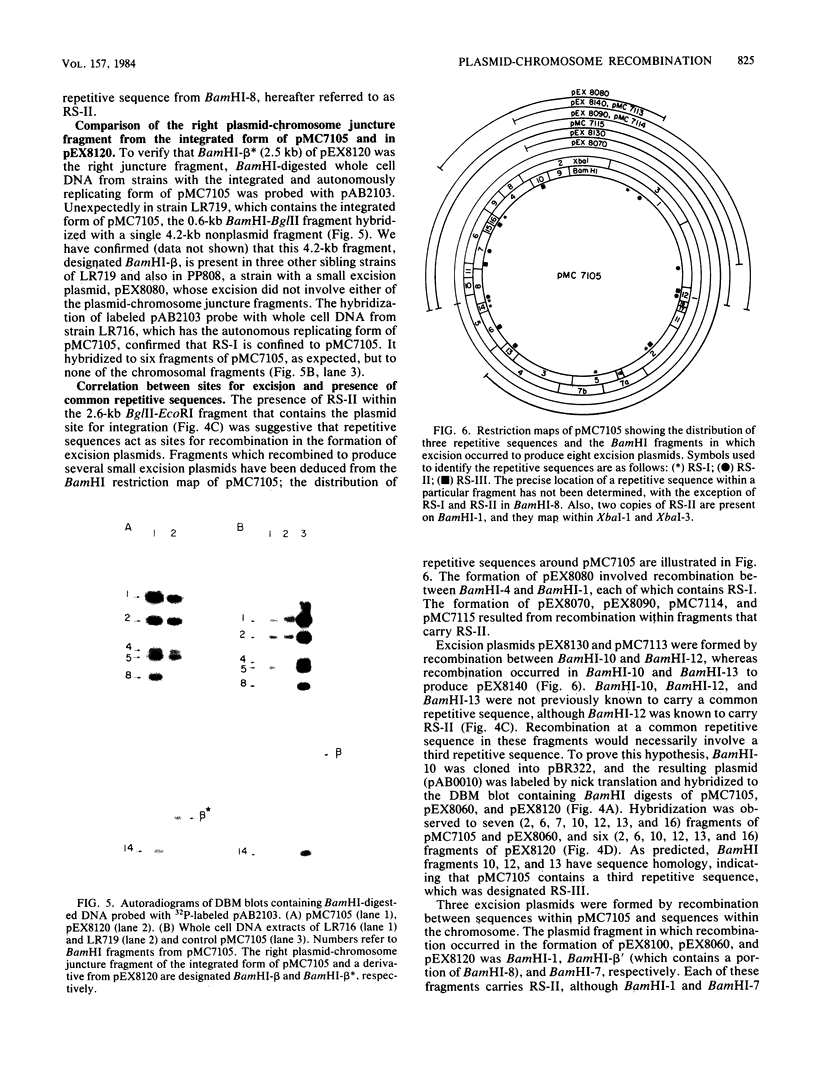
The site for integration of pMC7105 into the chromosome of Pseudomonas syringae pv. phaseolicola has been mapped to a 2.6-kilobase-pair (kb) Bg/II-EcoRI fragment on this 150-kb indigenous plasmid. Selected excision plasmids resulting from imprecise excision of pMC7105 were used to identify one of the plasmid-chromosome juncture fragments and to characterize the mechanism of recombination from the chromosome. A 14.2-kb BamHI plasmid-chromosome juncture fragment has been identified in pEX8060 (234 kb), an excision plasmid which carries approximately 90 kb of chromosomal sequences to the left of the site of integration. This fragment contains a portion of the 2.6-kb Bg/II-EcoRI fragment as well as chromosomal sequences. Blot hybridization with a probe made from selected fragments of pMC7105 revealed three distinct repetitive sequences, RS-I, RS-II, and RS-III, on this plasmid. The 2.6-kb fragment, to which the site of integration maps, also contains RS-II. Five copies of RS-II are present in pMC7105, and more than 20 copies are present in the chromosome. Eight small excision plasmids were shown to result from recombination among fragments of pMC7105 that contain common repetitive sequences. The results indicate that integration and excision of pMC7105 occur through general recombination at homologous repetitive sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Comai L., Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950–957. doi: 10.1128/jb.143.2.950-957.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Transposable element that causes mutations in a plant pathogenic Pseudomonas sp. J Bacteriol. 1983 Jun;154(3):1162–1167. doi: 10.1128/jb.154.3.1162-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., Mills D. Detection and characterization of plasmids in Pseudomonas glycinea. J Bacteriol. 1977 Jul;131(1):224–228. doi: 10.1128/jb.131.1.224-228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., Mills D. Integration and partial excision of a cryptic plasmid in Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1982 Nov;152(2):797–802. doi: 10.1128/jb.152.2.797-802.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G. IS2-IS2 and IS3-IS3 relative recombination frequencies in F integration. Plasmid. 1980 Jan;3(1):48–64. doi: 10.1016/s0147-619x(80)90033-5. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Mirels L. Excision of F plasmid sequences by recombination at directly repeated insertion sequence 2 elements: involvement of recA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3965–3969. doi: 10.1073/pnas.74.9.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. G., Deonier R. C. Specificity in the formation of delta tra F-prime plasmids. J Bacteriol. 1980 Aug;143(2):680–692. doi: 10.1128/jb.143.2.680-692.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Meulien P., Broda P. Identification of chromosomally integrated TOL DNA in cured derivatives of Pseudomonas putida PAW1. J Bacteriol. 1982 Nov;152(2):911–914. doi: 10.1128/jb.152.2.911-914.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Ohtsubo E., Zenilman M., Ohtsubo H., McCormick M., Machida C., Machida Y. Mechanism of insertion and cointegration mediated by IS1 and Tn3. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):283–295. doi: 10.1101/sqb.1981.045.01.041. [DOI] [PubMed] [Google Scholar]
- Timmons M. S., Bogardus A. M., Deonier R. C. Mapping of chromosomal IS5 elements that mediate type II F-prime plasmid excision in Escherichia coli K-12. J Bacteriol. 1983 Jan;153(1):395–407. doi: 10.1128/jb.153.1.395-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Purification and properties of the BamHI endonuclease. Methods Enzymol. 1980;65(1):147–153. doi: 10.1016/s0076-6879(80)65020-4. [DOI] [PubMed] [Google Scholar]