Abstract
The cistrons encoding the regulatory and catalytic polypeptides of aspartate transcarbamoylase (EC 2.1.3.2) from Escherichia coli K-12 have been cloned separately on plasmids from different incompatability groups. The catalytic cistron (pyrB) was carried by pACYC184 and expressed from its own promoter, whereas the regulatory cistron was expressed from the lac po of pBH20. The catalytic polypeptide chains assembled into enzymatically active trimers (c3) in vivo when expressed in the absence of regulatory subunits. Similarly, the regulatory polypeptide chains assembled into regulatory dimers (r2) in vivo in the absence of catalytic subunits. When cellular extracts containing regulatory dimers and catalytic trimers synthesized in separate cells were combined in vitro, partial spontaneous holoenzyme assembly occurred. When pyrB and pyrI were expressed from transcriptionally independent cistrons in the same cell, all detectable catalytic polypeptides were incorporated into the functional aspartate transcarbamoylase holoenzyme, 2(c3):3(r2). Thus, it is clear that the in vivo assembly of ATCase holoenzyme is a direct, spontaneous process involving the association of preformed regulatory subunits (r2) and catalytic subunits (c3). This procedure provides a general method for the construction of hybrid aspartate transcarbamoylase in vivo and may be applicable to other oligomeric enzymes constructed from different polypeptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bothwell M. A., Schachman H. K. A model for the assembly of aspartate transcarbamoylase from catalytic and regulatory subunits. J Biol Chem. 1980 Mar 10;255(5):1971–1977. [PubMed] [Google Scholar]
- Bothwell M. A., Schachman H. K. Equilibrium and kinetic studies of the association of catalytic and regulatory subunits of aspartate transcarbamoylase. J Biol Chem. 1980 Mar 10;255(5):1962–1970. [PubMed] [Google Scholar]
- Bothwell M., Schachman H. K. Pathways of assembly of aspartate transcarbamoylase from catalytic and regulatory subunits. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3221–3225. doi: 10.1073/pnas.71.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. L., Schachman H. K. Assembly of the catalytic trimers of aspartate transcarbamoylase from folded monomers. J Biol Chem. 1982 Aug 10;257(15):8638–8647. [PubMed] [Google Scholar]
- Burns D. L., Schachman H. K. Assembly of the catalytic trimers of aspartate transcarbamoylase from unfolded polypeptide chains. J Biol Chem. 1982 Aug 10;257(15):8648–8654. [PubMed] [Google Scholar]
- Burns D. L., Schachman H. K. Ligand-promoted strengthening of interchain bonding domains in catalytic subunits of aspartate transcarbamoylase. J Biol Chem. 1982 Oct 25;257(20):12214–12218. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. W. On the mechanism of assembly of the aspartate transcarbamoylase from Escherichia coli. Eur J Biochem. 1978 Oct;90(2):271–281. doi: 10.1111/j.1432-1033.1978.tb12600.x. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Crawford J. L., Monaco H. L., Ladner J. E., Ewards B. F., Evans D. R., Warren S. G., Wiley D. C., Ladner R. C., Lipscomb W. N. Crystal and molecular structures of native and CTP-liganded aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1982 Sep 15;160(2):219–263. doi: 10.1016/0022-2836(82)90175-9. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Lipscomb W. N. Interactions of phosphate ligands with Escherichia coli aspartate carbamoyltransferase in the crystalline state. J Mol Biol. 1982 Sep 15;160(2):265–286. doi: 10.1016/0022-2836(82)90176-0. [DOI] [PubMed] [Google Scholar]
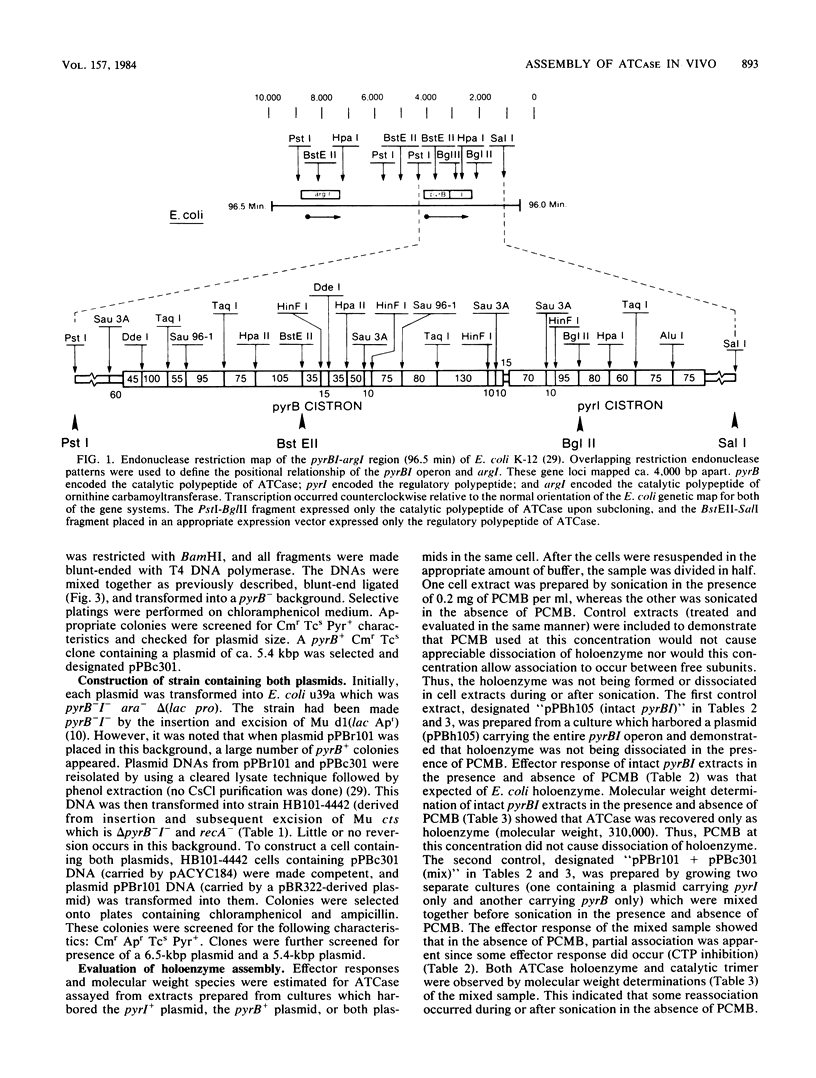
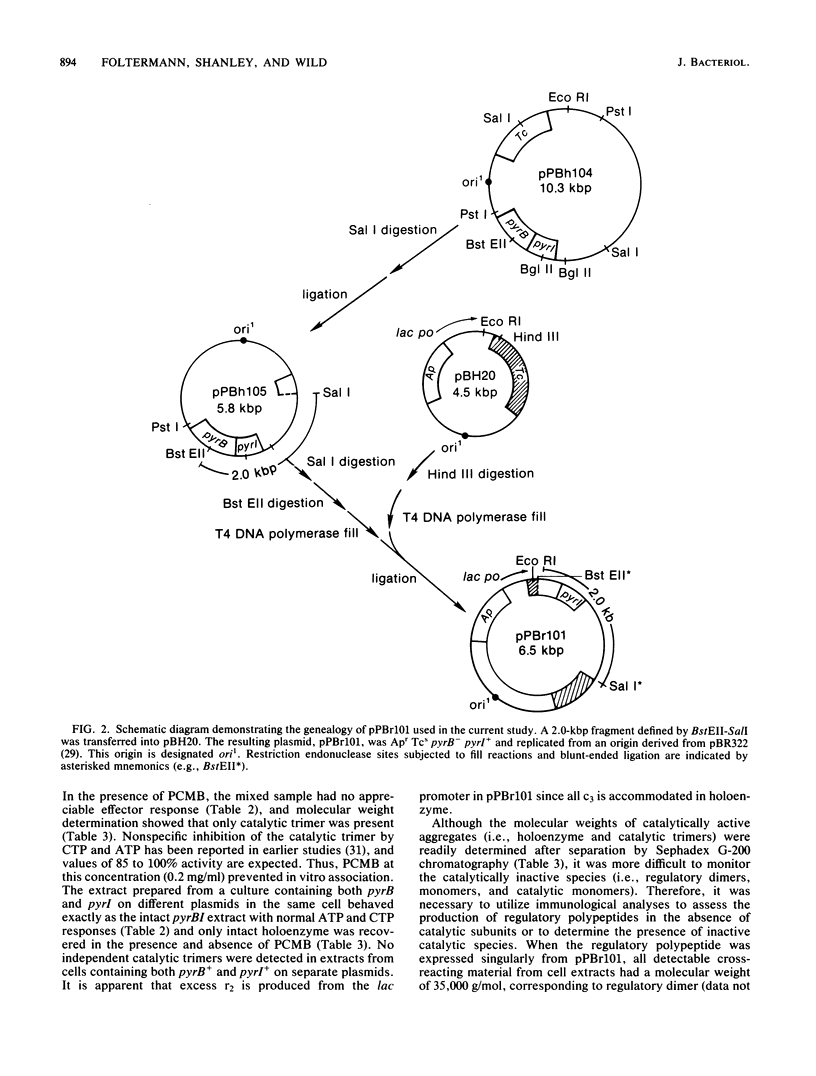
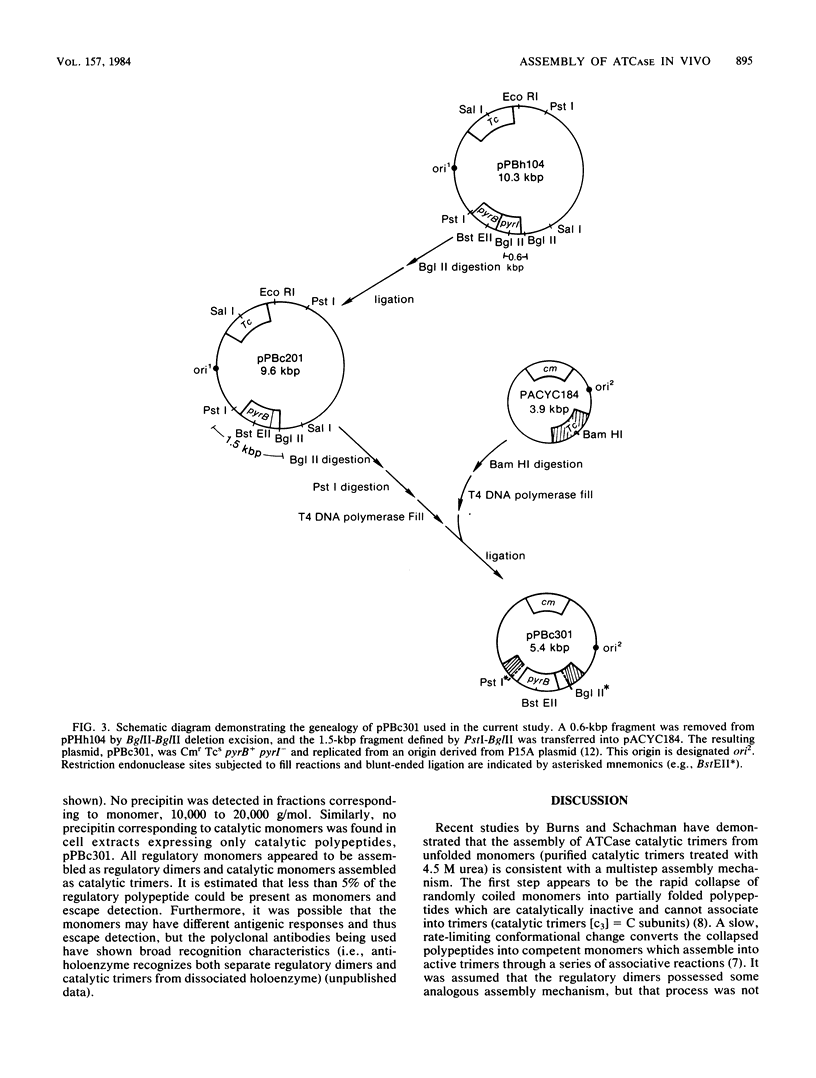
- Hoover T. A., Roof W. D., Foltermann K. F., O'Donovan G. A., Bencini D. A., Wild J. R. Nucleotide sequence of the structural gene (pyrB) that encodes the catalytic polypeptide of aspartate transcarbamoylase of Escherichia coli. Proc Natl Acad Sci U S A. 1983 May;80(9):2462–2466. doi: 10.1073/pnas.80.9.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarthy M. P., Allewell N. M. Thermodynamics of assembly of Escherichia coli aspartate transcarbamoylase. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6824–6828. doi: 10.1073/pnas.80.22.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Schachman H. K. Synthesis of aspartate transcarbamoylase in Escherichia coli: transcriptional regulation of the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1207–1211. doi: 10.1073/pnas.80.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Karels M. J., Navre M., Schachman H. K. Genes encoding Escherichia coli aspartate transcarbamoylase: the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4020–4024. doi: 10.1073/pnas.79.13.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof W. D., Foltermann K. F., Wild J. R. The organization and regulation of the pyrBI operon in E. coli includes a rho-independent attenuator sequence. Mol Gen Genet. 1982;187(3):391–400. doi: 10.1007/BF00332617. [DOI] [PubMed] [Google Scholar]
- Subramani S., Bothwell M. A., Gibbons I., Yang Y. R., Schachman H. K. Ligand-promoted weakening of intersubunit bonding domains in aspartate transcarbamolylase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3777–3781. doi: 10.1073/pnas.74.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter P., Rosenbusch J. P. Asymmetry of binding and physical assignments of CTP and ATP sites in aspartate transcarbamoylase. J Biol Chem. 1977 Nov 25;252(22):8136–8141. [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Hicks K. L., Donahue J. P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 Jan;80(2):368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr Regulation of Escherichia coli aspartate transcarbamylase synthesis by guanosine tetraphosphate and pyrimidine ribonucleoside triphosphates. J Bacteriol. 1983 Feb;153(2):998–1007. doi: 10.1128/jb.153.2.998-1007.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Wild J. R., Foltermann K. F., O'Donovan G. A. Regulatory divergence of aspartate transcarbamoylases within the enterobacteriaceae. Arch Biochem Biophys. 1980 May;201(2):506–517. doi: 10.1016/0003-9861(80)90539-1. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Kirschner M. W., Schachman H. K. Aspartate transcarbamoylase (Escherichia coli): preparation of subunits. Methods Enzymol. 1978;51:35–41. doi: 10.1016/s0076-6879(78)51007-0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Schachman H. K. Communication between catalytic subunits in hybrid aspartate transcarbamoylase molecules: effect of ligand binding to active chains on the conformation of unliganded, inactive chains. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5187–5191. doi: 10.1073/pnas.77.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]


