Abstract
Objectives.
Because many individuals with Mild Cognitive Impairment (MCI) will progress to a dementia diagnosis, this population is at high risk for losing functional independence. We examine trajectories of change in everyday function for individuals with cognitive deficits suggestive of MCI.
Design.
We utilized data from the longitudinal, multi-site Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, which allowed for post-hoc classification of MCI status at baseline using psycho metric definitions for amnestic MCI, non-amnestic MCI, multi-domain MCI, and no MCI.
Setting.
Six U.S. cities.
Participants.
2832 volunteers (mean age 74 years; 26% African American) living independently, recruited from senior housing, community centers, and hospitals and clinics.
Measurements.
Mixed effect models examined changes in self-reported instrumental and basic activities of daily living (IADLs and ADLs) from the MDS Home Care Interview in 2,358 participants over a three-year period.
Results.
In models for IADL performance, IADL difficulty, and a Daily Functioning Composite, there was a significant time by MCI classification interaction for each MCI subtype, indicating that all MCI groups showed faster rates of decline in everyday function relative to cognitively normal participants with no MCI.
Conclusion.
Results demonstrate the importance of MCI as a clinical entity that not only predicts progression to dementia but also predicts functional declines in activities that are key to autonomy and quality of life. MCI classification guidelines should allow for functional changes in MCI, and clinicians should monitor for such changes. Preservation of function may serve as a meaningful outcome for intervention efforts.
Keywords: Mild Cognitive Impairment, functional change, ADL, IADL
INTRODUCTION
Functional skills such as driving, handling personal finances, managing health care and medication regimens, and preparing nutritious meals are multidimensional, cognitively demanding tasks that are critical to independent living.1-6 These skills are likely to suffer when dementia-related cognitive deficits are experienced. Because many individuals with Mild Cognitive Impairment (MCI) will progress to a dementia diagnosis, this population is at high risk for losing functional independence.
MCI is widely viewed as a transitional syndrome leading to dementia. Clinical guidelines for determination of MCI have emerged in recent years7,8 and continue to be refined, but there is no consensus agreement on a single set of criteria for MCI other than presence of cognitive impairment of insufficient severity to constitute dementia.9,10 Recently, it has been recognized that MCI can be characterized by cognitive deficits broadly classified as amnestic (memory) and/or non-amnestic (e.g., executive function, abstract reasoning, language, or perceptual speed),10-12 which, in turn, may reflect multiple and often comorbid pathologies of neurodegenerative, vascular, metabolic, or traumatic origin. Primary amnestic deficits are presumed to most commonly represent preclinical Alzheimer’s disease (AD) or vascular dementia (VaD), while primary non-amnestic deficits—often executive or visuoperceptual—are presumed to represent preclinical frontotemporal dementia, Lewy Body dementia, Parkinson’s disease with dementia, or progressive supranuclear palsy, as well as AD or VaD. Implicit in these assumptions is that most MCI subtypes will evolve into recognized clinical entities,11 that both cerebrovascular and neurodegenerative pathologies are common,13 and that MCI subtypes involve cognitive symptoms12 that can affect everyday functioning. Further research is warranted regarding which MCI subtypes are most predictive of future decline in daily functioning.
A controversy in the conceptualization of MCI is the original stipulation that daily functioning must remain “intact” or “without disability.”7,14 Since MCI often reflects a transitional state between normal cognitive aging and mild dementia, there likely exists a continuum of decline in functional abilities, such that the relatively preserved abilities associated with normal aging begin to be affected by the cognitive changes experienced in MCI before reaching the level of functional impairment that is recognized as a defining feature of dementia. Individuals with MCI are likely to experience a loss of functional skills that lies somewhere between the subtle decrements associated with normal aging and the frank deficits associated with a dementia such as AD.
In the present analysis, we sought to examine trajectories of change in everyday function for individuals with cognitive deficits suggestive of different subtypes of MCI. We utilized data from the longitudinal Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study,15,16 which allowed for post-hoc classification of MCI status at baseline using psychometric definitions for amnestic MCI, non-amnestic MCI, and multi-domain MCI. We examined changes in self-reported instrumental and basic activities of daily living (IADLs and ADLs) over a three-year period. We predicted that at baseline, individuals with MCI would have more difficulty with these activities than cognitively normal participants. We expected that differences in everyday function between MCI groups and cognitively normal participants would grow over the three-year period, such that slopes of decline in function would be steeper over time for individuals with any form of MCI than for those without MCI, after accounting for potential confounds. Within the MCI groups, we expected that individuals with multi-domain MCI would experience steeper declines in everyday function than either of the single-domain MCI groups.
METHODS
Subjects
Participants were members of the ACTIVE study, a multi-site, controlled trial designed to investigate whether a diverse group of older adults randomized to one of three types of cognitive training could improve or maintain their daily living skills relative to a no-contact control group.15 For each ACTIVE participant, the main trial consisted of baseline cognitive assessment followed by randomization to one of the cognitive training groups or the control group, post-testing, annual booster training for a subsample, and annual post-tests.16 The ACTIVE study recruited older adults who were largely independent of formal care. Inclusion criteria for ACTIVE were: age ≥65 years; MMSE score ≥ 23; visual acuity > 20/50; no dependence in hygiene, bathing and dressing; no diagnosis of Alzheimer’s Disease; no stroke in the previous 12 months; no diagnosis of life-limiting cancer within the last 5 years; no current chemotherapy or radiation treatment; not residing in an institutional setting; available for the duration of the study; and no communication problems. The sample of 2,832 randomized participants thus consisted of relatively healthy older adults with a mean age of 74 years at enrollment. This research was conducted, and written informed consent was obtained, in compliance with the ethical rules for human experimentation stated in the Declaration of Helsinki and with the approval of the institutional review boards of all participating institutions.
Measures
Table 1 displays the psychometric measures constituting the three cognitive composites (memory, reasoning, processing speed) used to classify participants into MCI subgroups or the cognitively normal group according to their baseline performance. Functional status was assessed at baseline and three annual follow-up visits with a daily functioning composite derived from three scores representing self-reported ADL and IADL functioning from the Minimum Data Set-Home Care interview.17,18
Table 1.
Cognitive Composites Used to Classify Mild Cognitive Impairment (MCI) and Cognitively Normal Groups According to Baseline Performance*
| Cognitive Composite | Component Measures |
|---|---|
| Memory | Hopkins Verbal Learning Test36 |
| Auditory Verbal Learning Test37 | |
| Rivermead Behavioral Memory Test38 | |
| Reasoning | Word series39 |
| Letter series40 | |
| Letter sets40 | |
| Processing speed | Useful Field of View41-42 |
| Task 2: Divided attention | |
| Task 3: Selective attention | |
| Task 4: Complex attention |
Baseline performance at or below the 7th percentile (˜1.5 s.d. below the ACTIVE sample mean) on one or more of the cognitive composites resulted in MCI classification. Those with memory deficit only were classified amnestic MCI; those with reasoning or processing speed deficit were classified non-amnestic MCI; and those with two or three composite deficits were classified multi-domain MCI.
ADL performance was assessed with queries such as, “In the last 7 days, how much of the activity [e.g., combing/brushing hair] did you do on your own?” Raw score response codes ranged from 0 (independent) to 4 (total dependence). IADL performance was assessed with identically worded queries involving activities such as planning meals, handling money and checks, and keeping track of doctor appointments; raw score response codes ranged from 1 (did all on own) to 4 (fully performed by others). For both ADLs and IADLs, there also were response options indicating that a given activity had not occurred within the past 7 days. IADL difficulty was assessed with follow-up queries for each IADL, inquiring, “How difficult was it (or would it have been) to do on your own?” Responses ranged from 1 (not difficult) to 3 (great difficulty). Total scores on each scale were computed by taking the mean of all item responses. Everyday abilities also were assessed directly in the ACTIVE study with laboratory performance measures; these outcomes will be the focus of a separate manuscript.
Statistical Procedures
Blom transformations were used in the ACTIVE study to produce normally distributed scores on each cognitive test.16,19 These test scores were then summed to form baseline cognitive composite scores which were standardized to the mean and standard deviation of the entire study sample. Each cognitive composite score represented the average of 2 or 3 equally weighted test scores measuring the cognitive construct of interest (Table 1), thereby avoiding test-specific definition of cognitive deficits. Participants with relative deficits (≤7th percentile) were identified in each of three cognitive domains (memory, reasoning, and speed of processing), resulting in classification to one of the three mutually exclusive MCI subgroups described below or to the cognitively normal group.
Total scores on the MDS scales and the daily functioning composite were similarly normalized. Scores at each time point were pooled and standardized to the baseline mean and standard deviation of the entire study sample.
Psychometric criteria for MCI were defined as follows: baseline performance at or below the 7th percentile (approximately 1.5 standard deviations below ACTIVE sample means) on one or more of the three cognitive composites. Thus, a given participant might have no composite cognitive deficits (cognitively normal group for the current analyses), a deficit on either the memory, reasoning, or processing speed composite (single-domain MCI, further conceptualized as single-domain amnestic [memory] MCI or single-domain non-amnestic [reasoning or processing speed] MCI), or deficits on more than one cognitive composite (multi-domain MCI).
Of the 2,832 older adults enrolled in ACTIVE at baseline, 30 were inappropriately randomized and dropped from the study. Of the remaining 2,802 participants, 2,777 had cognitive data that enabled MCI classification, and 2,454 of these provided data for one or more of the first three annual follow-up visits. Among these participants, our criteria for MCI yielded a sample of 344 participants (14.0% of the sample) with possible MCI at enrollment (84, or 3.4%, single-domain amnestic; 171, or 7.0%, single-domain non-amnestic; and 89, or 3.6%, multi-domain MCI). The remaining 2,110 participants (86.0% of this sample) constituted the cognitively normal reference group for the present study. Analyses focused on differences in baseline functional status as well as functional trajectories across the four groups.
Mixed-effects models conducted in SAS procedure MIXED (SAS Institute, 2000, Cary, NC) were used to test whether differences in trajectories of change in everyday function could be predicted by MCI classifications. For these models, we omitted individuals who might have unrecognized dementia by excluding all participants within the MCI groups whose baseline functional status was ≥1.5 S.D. poorer than the full sample means on any functional measure. These mixed models examined changes in everyday function among the remaining 2,358 participants (56 amnestic, 133 non-amnestic, 59 multi-domain, and 2,110 no MCI) across three annual assessments following study enrollment by fitting a separate regression curve across available longitudinal assessments for each individual participant, and then aggregating the parameters of these growth curves and testing them as a function of group. This technique enabled us to include all participants who provided data at one or more annual follow-up assessments, thereby allowing inclusion of participants who missed a visit or dropped out of the study before the third annual assessment. We modeled individual growth curves as a function of MCI classification, time (years from baseline), baseline function, age, gender, education, visual acuity, and training group. Baseline function, age, education, and visual acuity were entered as mean-centered covariates in these models.
RESULTS
The 2,454 participants included in this analysis were not different from the participants with no follow-up data in terms of scores on the daily functioning composite or visual acuity (Ps > .05). However, male gender, older age, and meeting psychometric criteria for any MCI classification were associated with reduced likelihood of having follow-up data (Ps < .05). By MCI classification, 74% of the amnestic MCI group, 71% of the non-amnestic MCI group, and 75% of the multi-domain MCI group were retained at the third annual assessment, compared to 86% of those not meeting criteria for MCI.
See Table 2 for results of bivariate analyses of demographic and functional characteristics of the study sample at baseline by MCI classification, along with p-values for differences from cognitively normal participants. Results demonstrated that all MCI groups were older, had lower MMSE scores, and had worse visual acuity than those without MCI, despite the fact study inclusion criteria ensured that mental status and vision were within ranges considered grossly intact. Amnestic MCI was associated with reports of poorer ADL performance than cognitively normal participants at baseline. Participants with non-amnestic and multi-domain MCI did not differ from those without MCI on reports of baseline ADL performance. All three MCI groups reported significantly greater baseline IADL difficulty than those without MCI, but the non-amnestic MCI group did not differ from those without MCI on reports of IADL performance. For the overall daily functioning composite, significantly poorer function was observed at baseline for all three MCI groups compared to cognitively normal participants. Adjustment for age, gender, education, and visual acuity did not change the ADL performance findings or the IADL difficulty findings but did eliminate baseline differences in IADL performance for all MCI groups, and in the daily functioning composite for the non-amnestic group only, relative to cognitively normal participants.
Table 2.
Descriptive Characteristics of the Sample at Baseline by MCI Classification
| Cognitively Normal | Amnestic MCI | Non-amnestic MCI | Multi-domain MCI | |
|---|---|---|---|---|
| Total n | 2110 | 84 | 171 | 89 |
| Age, mean (SD) | 72.9 (5.4) | 77.0 (7.0)*** | 76.5 (6.2)*** | 78.8 (6.6)*** |
| Gender (% female) | 77.4 | 65.5* | 84.2* | 57.3*** |
| Years of education, mean (SD) | 13.8 (2.6) | 12.6 (3.1)*** | 12.3 (2.4)*** | 11.8 (2.8)*** |
| MMSE, mean (SD) | 27.6 (1.8) | 26.0 (1.9)*** | 26.2 (2.1)*** | 25.1 (1.8)*** |
| 67.0 | 69.1 | |||
| Visual acuity†, mean (SD) | 74.0 (11.2) | (13.0)*** | (12.3)*** | 67.4 (11.9)*** |
| Everyday functioning‡ | ||||
| ADL performance, mean (SD) | 0.3 (0.9) | 0.6 (1.5)** | 0.3 (0.9) | 0.4 (0.8) |
| IADL performance, mean (SD) | 4.2 (4.8) | 5.3 (4.8)* | 4.2 (4.8) | 5.7 (6.2)** |
| IADL difficulty, mean (SD) | 1.2 (2.2) | 2.5 (3.5)*** | 1.8 (2.7)** | 2.5 (3.6)*** |
| Daily functioning composite, mean (SD) | −0.1 (2.0) | 0.9 (2.4)*** | 0.2 (2.2)* | 0.8 (2.5)*** |
Note: Asterisks denote significant differences for MCI classification categories relative to cognitively normal participants who constituted the reference category. *p < .05; **p < .01; ***p < .001
The ACTIVE15 scoring system was used to measure visual acuity, using participants’ corrective lenses if normally worn for distance vision. Resulting scores may range from 0 (Snellen score of 20/125) to 90 (Snellen score of 20/16); obtained mean scores of 67 to 74 correspond to Snellen scores of 20/27 to 20/23.
Higher scores on these four scales indicate more functional dependence and greater difficulty.
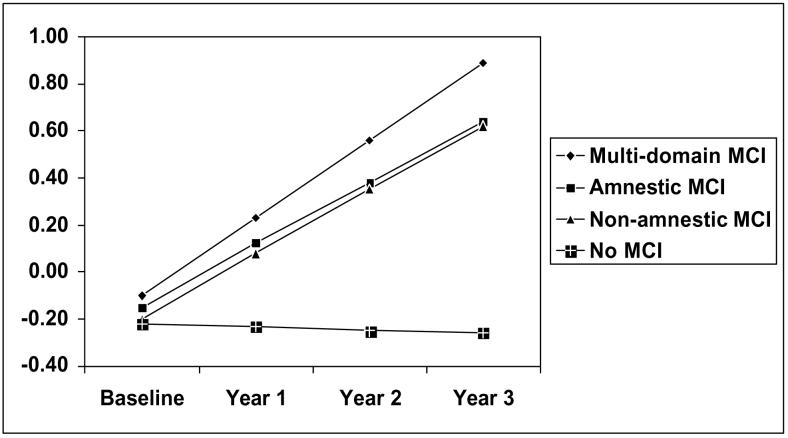
Longitudinal results obtained from mixed-effects models estimated separately for each functional outcome are presented in Table 3. These models excluded those in the MCI groups whose baseline score on any measure used to form the daily functioning composite was ≥ 1.5 SD worse than the mean score of the full sample. The interaction of time by MCI classification constituted the test of the main hypothesis. In models for IADL performance, IADL difficulty, and the daily functioning composite, there was a significant time by MCI classification interaction for each MCI subtype, indicating that all MCI groups showed faster rates of decline in everyday function relative to cognitively normal participants. For ADL performance, only non-amnestic MCI was a significant predictor of change. Figure 1 illustrates the relationship between MCI classification and change in the daily functioning composite over the three-year period of follow-up. We also ran supplementary analyses in which we controlled for the 3-way interaction of MCI by time by training group; MCI group remained a significant predictor of everyday function trajectories in each model.
Table 3.
Models† of Longitudinal Change in Everyday Function from Baseline to 3 Years Later
| ADL performance | IADL performance | IADL difficulty | Daily Functioning composite | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Intercept | 0.212*** | 0.062 | 5.469*** | 0.213 | 1.309*** | 0.129 | 0.171 | 0.941 |
| Time | 0.036** | 0.014 | 0.030 | 0.032 | 0.033 | 0.021 | −0.015 | 0.016 |
| Group‡ | ||||||||
| Amnestic | ||||||||
| MCI | −0.036 | 0.101 | 0.186 | 0.278 | 0.073 | 0.187 | 0.068 | 0.141 |
| Non-amnestic | ||||||||
| MCI | −0.062 | 0.067 | 0.003 | 0.183 | 0.012 | 0.123 | 0.019 | 0.093 |
| Multi-domain | ||||||||
| MCI | −0.039 | 0.010 | 0.343 | 0.276 | 0.271 | 0.185 | 0.114 | 0.140 |
| Group × Time | ||||||||
| Amnestic | ||||||||
| MCI × Time | 0.111 | 0.089 | 0.668** | 0.204 | 0.391** | 0.135 | 0.278** | 0.102 |
| Non-amnestic | ||||||||
| MCI × Time | 0.216*** | 0.058 | 0.319* | 0.133 | 0.410*** | 0.088 | 0.288*** | 0.066 |
| Multi-domain | ||||||||
| MCI × Time | 0.107 | 0.086 | 0.509** | 0.197 | 0.372** | 0.130 | 0.346*** | 0.098 |
Note. *p < .05; **p < .01; ***p < .001
All models included group, time, group × time, and the following covariates: baseline everyday function (centered ADL performance, IADL performance, IADL difficulty, or the Daily Functioning composite in the respective models), age (centered), gender, years of education (centered), visual acuity (centered), and training group.
Cognitively normal participants were the reference group.
Figure 1.
[v1] Covariate-adjusted Levels of Reported Dependence/difficulty on the Minimum Data Set Daily Functioning Composite by MCI Classification and Time. Higher Scores Denote More Difficulty.
DISCUSSION
The primary finding of this study is that older adults with both amnestic and non-amnestic cognitive deficits consistent with MCI show significantly steeper declines in everyday function over a three-year period than individuals with normal cognitive profiles. These results demonstrate the importance of MCI as a clinical entity that not only predicts progression to dementia but also predicts functional declines in activities that are key to autonomy and quality of life, even in the absence of dementia or prior to diagnosis. The study extends other recent evidence supporting this contention20,21 by demonstrating meaningful functional decrements in MCI that grow in magnitude over time across a range of daily activities. These results support revised classification guidelines that allow for functional changes in MCI that fall short of functional dependence.
Given population base rates of MCI ranging from 5 to 25%,22-24 it is highly likely that a portion of ACTIVE participants would meet clinical MCI criteria (e.g., Mayo25 or modified Mayo criteria10) if explicitly evaluated for case identification. Although the ACTIVE study did not include clinical determination of MCI, the inclusion of a number of cognitive function measures allowed us to identify individuals who met psychometric criteria for MCI classification. While we acknowledge that there are limitations to this approach, the psychometric classification provided useful data concerning the prediction of decrements in important everyday activities.
Considering the variable stability in MCI classification reported in the literature,24,26 an alternative to our baseline-only classification of MCI would have been to reclassify each participant annually, based upon available cognitive profiles at each follow-up assessment. However, given the selective attrition of participants who had cognitive deficits consistent with MCI at baseline, those participants with MCI who were retained in all subsequent years might represent only the earliest stage and/or highest functioning members of the baseline MCI group. Furthermore, longitudinal stability of MCI classification in the ACTIVE sample has been explicitly examined over a two-year period 27 and found to be stable in 86% of cases, while the remaining 14% of “unstable” classifications represented a mixed group of individuals whose cognitive profiles either “reverted” to normal, consistent with several prior studies (e.g.28), or progressed from a single-domain deficit to a multi-domain MCI classification.27 Our statistical approach allowed us to examine changes in everyday function among all participants who completed one or more longitudinal assessments, despite significantly lower retention rates of individuals with psychometric characteristics of MCI at enrollment. In the context of selective attrition that would serve to bias our results toward the null, our findings of functional decline in MCI can be considered fairly robust.
On the overall composite of daily functioning comprising one index of ADL function and two indices of IADL function, significantly poorer global function was observed at baseline for all MCI subtypes. However, results specific to the component indices of function included the unexpected finding of ADL decrements at baseline in amnestic MCI. Self-care deficits are anticipated in mid-stage dementia but not in a preclinical syndrome such as MCI. It is important to note that statistically lower function among the amnestic MCI group relative to cognitively normal participants in ACTIVE may denote subtle ADL changes that do not rise to the level of frank impairment. On the other hand, some individuals meeting psychometric criteria for the MCI designation, particularly those with memory deficits, may represent undiagnosed AD. In such a scenario, ADL deficits would be consistent with the diagnosis. Tabert and colleagues29 found that patients with deficits in memory plus other cognitive deficits were at highest risk for progression to AD. This “memory plus” designation corresponds to a subset of our multi-domain MCI group; the remainder of that group was composed of participants with two non-amnestic deficits (i.e., in processing speed and reasoning) but no memory deficit. Taken together, these results underscore the potential role of memory impairment as a correlate of lower function in the realm of basic ADL functioning.29
In order to minimize the possibility that our sample included individuals with unrecognized dementia, we restricted our longitudinal models of functional change to participants who met not only ACTIVE inclusion criteria but also an empirical definition of intact function at baseline. Despite this restriction, all MCI groups declined in IADL performance, reported increasing IADL difficulty, and declined on the daily functioning composite. However, only the non-amnestic MCI group demonstrated longitudinal declines in ADL performance. This latter finding complements the finding of poorer ADL function in amnestic MCI at baseline and confirms the importance of executive abilities such as speed and reasoning to ADL performance, a result that has been reported in previous cross-sectional30 and longitudinal31 research.
This study also provides some evidence that self report can remain a valuable source of information for those with MCI, despite the risk of compromised insight among those with encroaching dementia.32 Individuals with cognitive deficits at baseline reported greater IADL difficulty and lower levels of independence on a global composite of daily functioning, and their reports of functional difficulties grew in magnitude over time, suggesting insight regarding their functional changes. This implication is consistent with another recent analysis based on the ACTIVE sample, in which individuals with memory deficits suggestive of amnestic MCI demonstrated insight into their cognitive declines,33 as well as a recent report of heightened insight in individuals with MCI with regard to their memory functioning.34 The self-reported functional data examined in this paper will be augmented by future research examining objective, laboratory-based performance of IADL function over time in MCI.
It is important to note that while our examination of functional status over time controlled for the training and control group assignments of each participant in the ACTIVE study, we did not explore the impact of each of the ACTIVE training protocols on the everyday function of individuals meeting criteria for MCI, either as a group or by MCI subtype. The cognitive and functional responses of individuals with cognitive profiles suggestive of MCI to the memory, reasoning, and speed training arms of the ACTIVE study are of great interest for future intervention applications. Indeed, preliminary research35 has suggested differential cognitive gains associated with each of the training protocols among participants with amnestic deficits. Cognitive and functional responses to each training protocol, by MCI subtype, will be the focus of further examination in the near future.
The authors hope that the present study will help persuade clinicians to monitor their patients with MCI for anticipated functional changes and consider early intervention strategies. Potential functional losses in MCI also can serve as clinically meaningful outcomes for trials of pharmacologic and alternative interventions for MCI.
ACKNOWLEDGEMENTS
Financial Disclosures:
Authors receiving financial support for health-related research, consultantships, speakers forums, or company holdings or patents related to this research:
Wadley: none
Crowe: none
Marsiske: none
Cook: none
Unverzagt: none
Rosenberg: none
Rexroth: none
Author Contributions:
Study concept & design—Wadley, Crowe, Marsiske, Cook, Unverzagt, Rosenberg, Rexroth
Acquisition of subjects/data—Wadley, Crowe, Marsiske, Cook, Unverzagt, Rosenberg, Rexroth
Analysis & interpretation of data—Wadley, Crowe, Marsiske, Cook, Unverzagt
Preparation of manuscript—Wadley, Crowe, Marsiske, Cook, Unverzagt, Rosenberg, Rexroth
Sponsor’s Role:
ACTIVE was supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Rehabilitation Center for the Aged (U01 NR04507), Indiana University School of Medicine (U01 NR04508), Johns Hopkins University (U01 AG14260), New England Research Institutes (U01 AG14282), Pennsylvania State University (U01 AG14263), University of Alabama at Birmingham (U01 AG14289), and University of Florida (U01 AG014276).
REFERENCES
- 1.Hunt L, Murphy CF, Carr D, et al. Reliability of the Washington University road test: A performance-based assessment for drivers with dementia of the Alzheimer type. Arch Neurol. 1997;54:707–712. doi: 10.1001/archneur.1997.00550180029008. [DOI] [PubMed] [Google Scholar]
- 2.Johansson K, Lundberg C. The 1994 international consensus conference on dementia and driving: A brief report. Alz Dis Assoc Disorders. 1997;11:62–69. doi: 10.1097/00002093-199706001-00013. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg C, Johansson K, Ball K, et al. Dementia and driving: An attempt at consensus. Alz Dis Assoc Disorders. 1997;11:28–37. doi: 10.1097/00002093-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo M, Reinach S, McGehee D, et al. Simulated car crashes and crash predictors in drivers with Alzheimer's disease. Arch Neurol. 1997;54:545–551. doi: 10.1001/archneur.1997.00550170027011. [DOI] [PubMed] [Google Scholar]
- 5.Aguero-Torres H, Fratiglioni L, Guo Z, et al. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88:452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauvaget C, Yamada M, Fujiwara S, et al. Dementia as a predictor of functional disability: A four-year follow-up study. Gerontology. 2002;48:226–233. doi: 10.1159/000058355. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in Mild Cognitive Impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Early detection of dementia: Mild Cognitive Impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC. Mild cognitive impairment clinical trials. Nature Reviews/ Drug Discovery. 2003;2:650–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Internal Med. 2004;256:1–12. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Mayeux R. Mild Cognitive Impairment: Risk factors, etiology and biomarkers. 3rd Annual Mild Cognitive Impairment (MCI) Symposium; Miami, Florida. 2005. Paper presented at. [Google Scholar]
- 12.Loewenstein DA, Acevedo A, Agron J, et al. Cognitive profiles in Alzheimer’s disease and in mild cognitive impairment of different etiologies. Dementia, Geriatrics, and Cognitive Disorders. 2006;21:309–315. doi: 10.1159/000091522. [DOI] [PubMed] [Google Scholar]
- 13.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 15.Jobe JB, Smith DM, Ball K, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults. A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: Applicability of the MDS-HC. JAGS. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 18.Morris J, Morris S. ADL assessment measures for use with frail elders. In: Ory M, editor. Measurement in Elderly Chronic Care Populations. Springer; New York: 1997. [Google Scholar]
- 19.Blom G. Statistical Estimates and Transformed Beta Variables. John Wiley & Sons; New York: 1958. [Google Scholar]
- 20.Daly E, Zaitchik D, Copeland M, et al. Predicting conversion to Alzheimer disease using standardized clinical information. Archi Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 21.Griffith R, Belue K, Sicoloa A, et al. Impaired financial abilities in mild cognitive impairment. Neurology. 2003;60:449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- 22.Ebly EM, Hogan DB, Parhad IMI. Cognitive impairment in the nondemented elderly. Arch Neurol. 1995;52:612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 23.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 24.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 26.Ganguli M. Mild cognitive impairment and the 7 uses of epidemiology. Alz Dis Assoc Disorders. 2006;20:S52–S57. doi: 10.1097/00002093-200607001-00007. [DOI] [PubMed] [Google Scholar]
- 27.Cook SE, Marsiske M, Unverzaft FW, et al. Identification of Mild Cognitive Impairment in ACTIVE: classification and stability. Under review. [DOI] [PMC free article] [PubMed]
- 28.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 29.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 30.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 31.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older woment. J Gerontol B Pscyhol Sci. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 32.Honig LS, Mayeux R. Natural history of Alzheimer’s disease. Aging (Milano) 2001;13:171–182. doi: 10.1007/BF03351476. [DOI] [PubMed] [Google Scholar]
- 33.Crowe M, Andel R, Wadley V, et al. Subjective cognitive function and decline among older adults with psychometrically defined amnestic MCI. Int J Geriatr Psychiatry. 2006;21:1187–1192. doi: 10.1002/gps.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook S, Marsiske M. Subjective memory beliefs and cognitive performance I normal and mildly impaired older adults. Aging and Mental Health. 2006;10:413–423. doi: 10.1080/13607860600638487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unverzagt FW, Kasten L, Marsiske M, et al. Effect of memory impairment on training outcome in ACTIVE. Presented as part of a symposium, Results from the ACTIVE study: understanding the observed training effects, S Tennestedt, Chair, at the annual meetings of the Gerontological Society of America; Washington, DC. 2004. November. [Google Scholar]
- 36.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychology. 1991;5:125–142. [Google Scholar]
- 37.Rey A. L’examen psychologique dans les cas d’encephalopathie tramatique. Archives de Psychologie. 1941;28:21. [Google Scholar]
- 38.Wilson B, Cockburn J, Baddely A, et al. The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol. 1989;11:855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 39.Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Consulting Psychologists Press; Palo Alto, California: 1985. [Google Scholar]
- 40.Thurstone L, Thurstone T. Examiner Manual for the SRA Primary Abilities Test (Form 10−14) Science Research Associates; Chicago, Illinois: 1949. [Google Scholar]
- 41.Owsley C, Ball K, McGwin G, Jr, et al. Visual processing impairment and risk of motor vehicle crash among. older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 42.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Opt Assoc. 1993;64:71–79. [PubMed] [Google Scholar]