Abstract
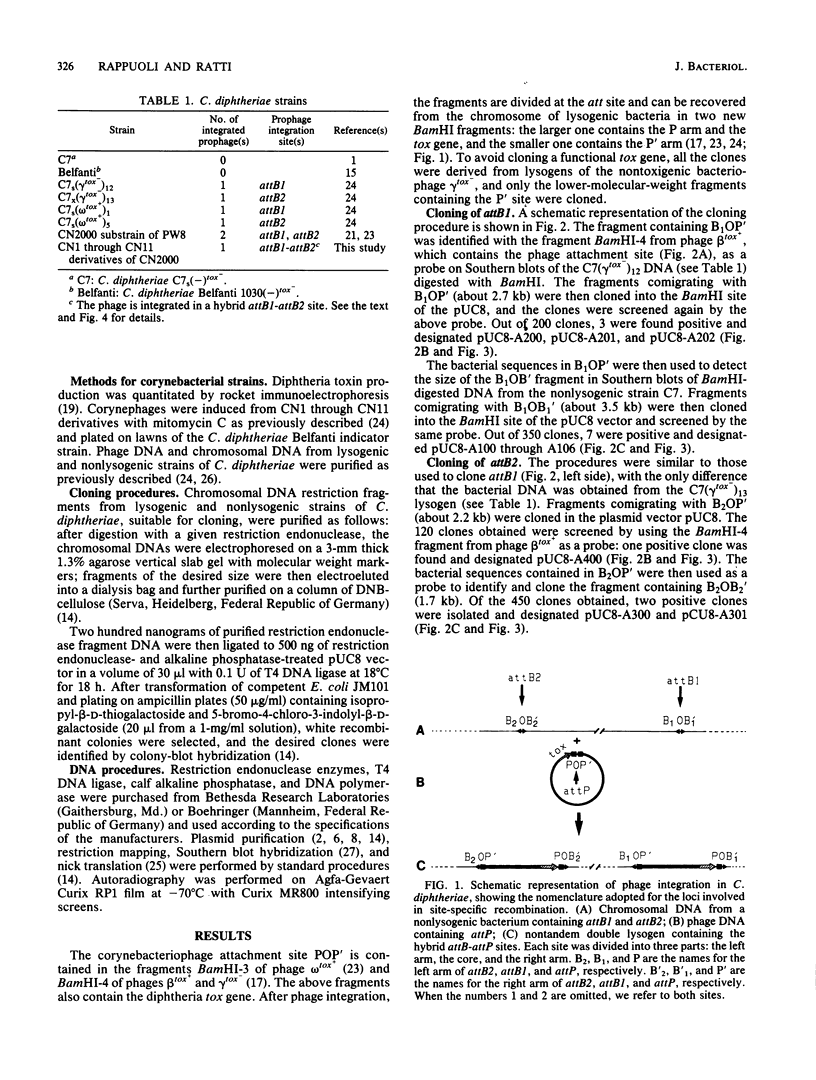
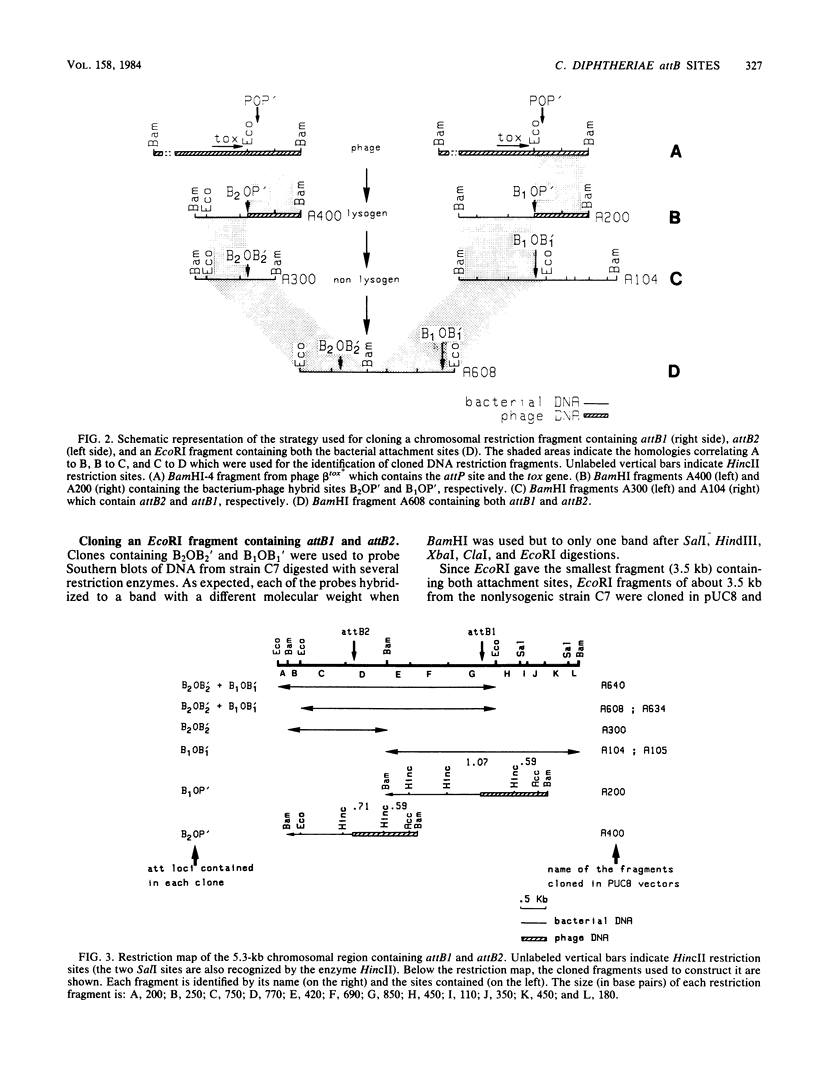
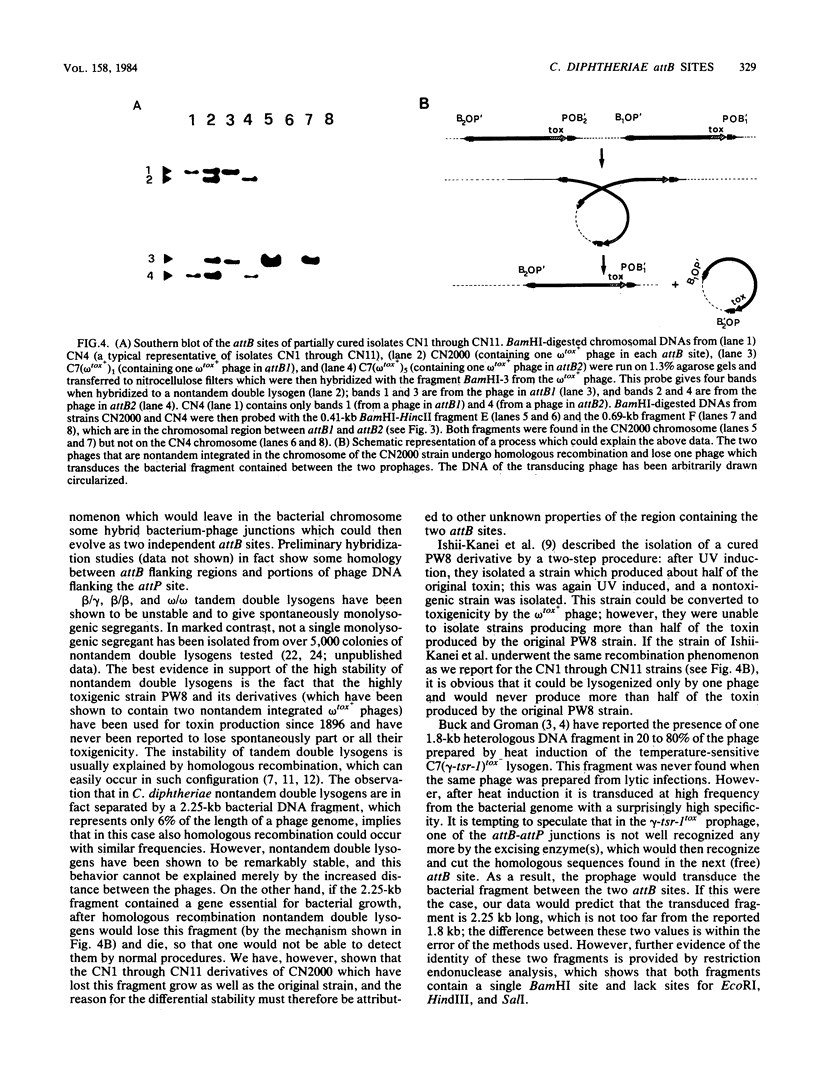
The chromosome of Corynebacterium diphtheriae C7 was recently shown to contain two equivalent attachment sites (attB1 and attB2) for lysogenization by corynephages (R. Rappuoli, J.L. Michel, and J.R. Murphy, J. Bacteriol. 153:1202-1210, 1983). Portions of bacterial chromosome containing each attB site, as well as a 3.5-kilobase (kb) EcoRI fragment containing both attB1 and attB2 sites, were cloned in the pUC8 plasmid vector. Restriction endonuclease mapping and Southern blot hybridization analysis of restriction endonuclease fragments showed that attB1 and attB2 are 2.25 kb apart on the chromosome. Furthermore, a 0.85-kb HincII-EcoRI restriction endonuclease fragment containing attB1, a 0.77-kb HincII-BamHI fragment containing attB2, and a 1.2-kb EcoRI-BamHI fragment containing attP share short homologous regions. No homology was detected between the sequences flanking the two attB sites. The isolation of a segregant which had lost the entire chromosomal segment contained between attB1 and attB2 suggests that this region is not essential for growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARDSDALE W. L., PAPPENHEIMER A. M., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. doi: 10.1128/jb.67.2.220-232.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Genetic elements novel for Corynebacterium diphtheriae: specialized transducing elements and transposons. J Bacteriol. 1981 Oct;148(1):143–152. doi: 10.1128/jb.148.1.143-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G., Groman N., Falkow S. Relationship between beta converting and gamma non-converting corynebacteriophage DNA. Nature. 1978 Feb 16;271(5646):683–685. doi: 10.1038/271683a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N., Laird W. Bacteriophage production by doubly lysogenic Corynebacterium diphtheriae. J Virol. 1977 Sep;23(3):592–598. doi: 10.1128/jvi.23.3.592-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ishii-Kanei C., Uchida T., Yoneda M. Isolation of a cured strain from Corynebacterium diphtheriae PW8. Infect Immun. 1979 Sep;25(3):1081–1083. doi: 10.1128/iai.25.3.1081-1083.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Orientation of the tox gene in the prophage of corynebacteriophage beta. J Virol. 1976 Jul;19(1):228–231. doi: 10.1128/jvi.19.1.228-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Prophage map of converting corynebacteriophage beta. J Virol. 1976 Jul;19(1):208–219. doi: 10.1128/jvi.19.1.208-219.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximescu P. New host-strains for the lysogenic Corynebacterium diphtheriae Park Williams No. 8 strain. J Gen Microbiol. 1968 Aug;53(1):125–133. doi: 10.1099/00221287-53-1-125. [DOI] [PubMed] [Google Scholar]
- Michel J. L., Rappuoli R., Murphy J. R., Pappenheimer A. M., Jr Restriction endonuclease map of the nontoxigenic corynephage gamma c and its relationship to the toxigenic corynephage beta c. J Virol. 1982 May;42(2):510–518. doi: 10.1128/jvi.42.2.510-518.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Rappuoli R. Isolation and characterization of Corynebacterium diphtheriae nontandem double lysogens hyperproducing CRM197. Appl Environ Microbiol. 1983 Sep;46(3):560–564. doi: 10.1128/aem.46.3.560-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Michel J. L., Murphy J. R. Integration of corynebacteriophages beta tox+, omega tox+, and gamma tox- into two attachment sites on the Corynebacterium diphtheriae chromosome. J Bacteriol. 1983 Mar;153(3):1202–1210. doi: 10.1128/jb.153.3.1202-1210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Michel J. L., Murphy J. R. Restriction endonuclease map of corynebacteriophage omega ctox+ isolated from the Park-Williams no. 8 strain of Corynebacterium diphtheriae. J Virol. 1983 Feb;45(2):524–530. doi: 10.1128/jvi.45.2.524-530.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schiller J., Groman N., Coyle M. Plasmids in Corynebacterium diphtheriae and diphtheroids mediating erythromycin resistance. Antimicrob Agents Chemother. 1980 Nov;18(5):814–821. doi: 10.1128/aac.18.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]



