Abstract
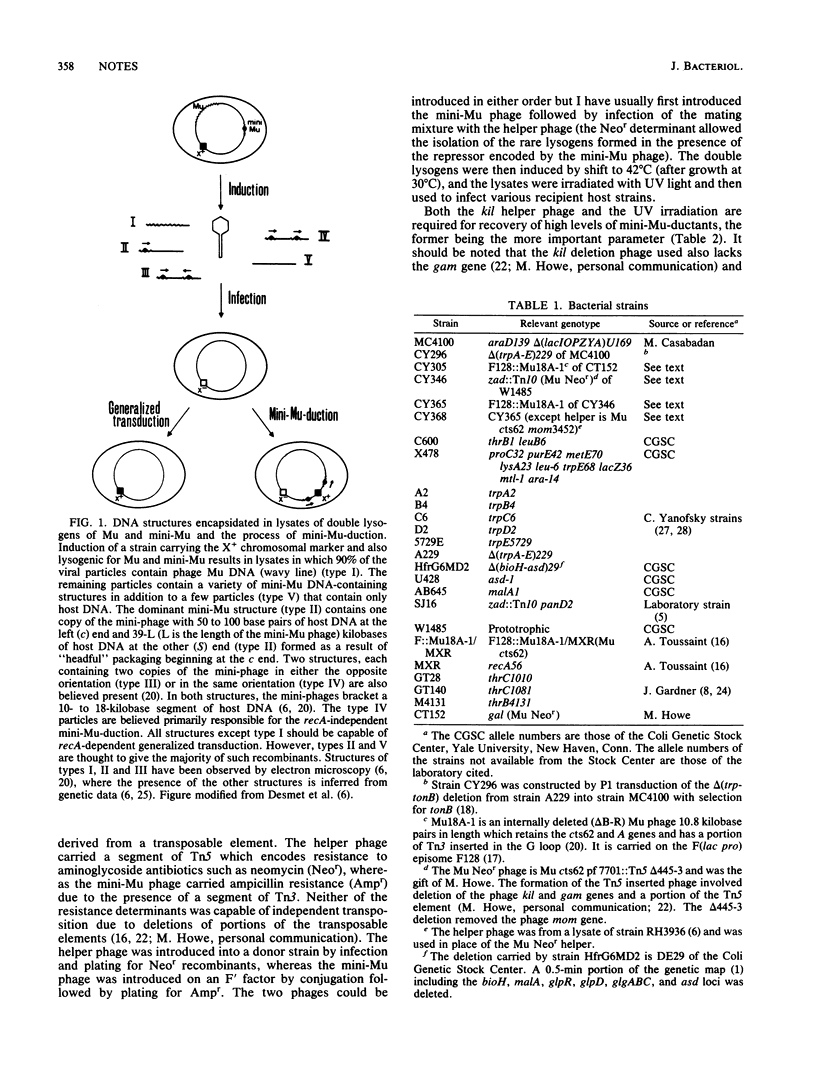
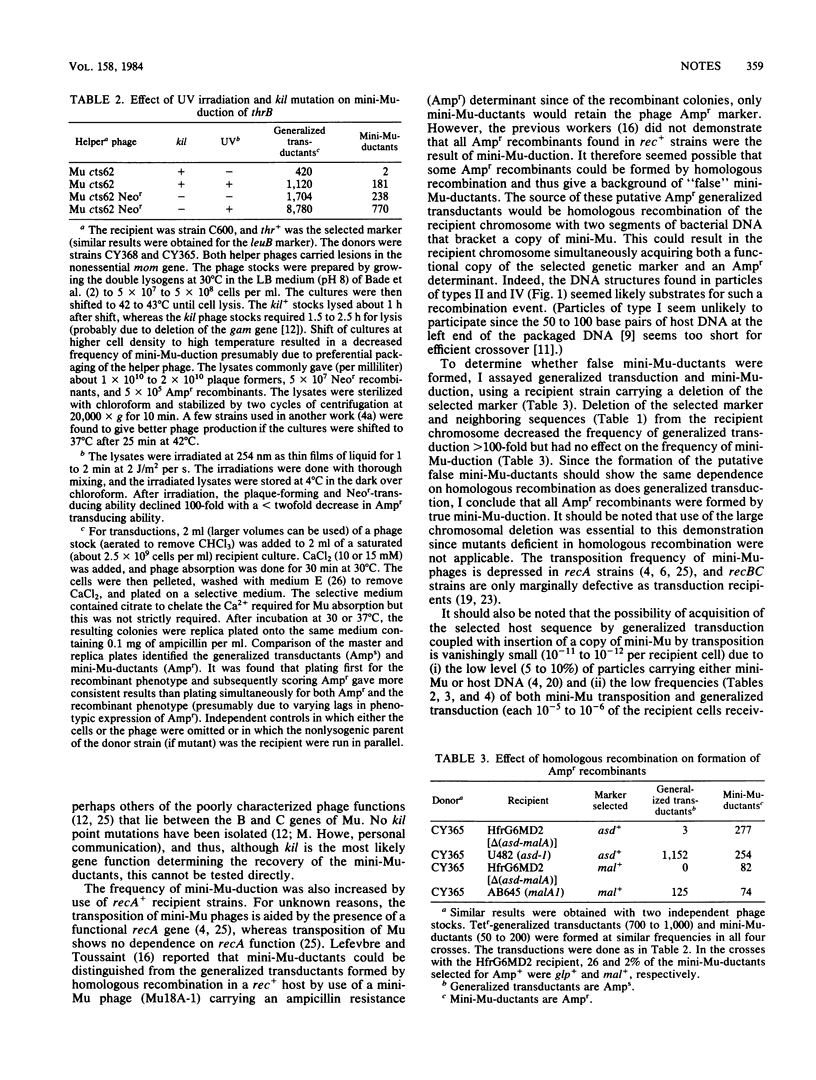
In mini-Mu-duction, segments of host DNA bracketed between two copies of an internally deleted Mu phage (a mini-Mu) can be packaged within Mu phage particles. Upon infection of a second host strain, the DNA injected by these particles can insert into the chromosomal DNA in a reaction catalyzed by the phage A gene product (transposase), which is independent of homologous recombination. This results in a partially diploid host strain in which the duplicated host DNA is bracketed by two copies of the mini-Mu phage (Faelen et al., Mol. Gen. Genet. 176:191-197, 1979). The frequency of mini-Mu-duction reported previously was low (10(-8) to 10(-9) per recipient cell) thus limiting its use to rather stable mutational lesions. I have increased the frequency of mini-Mu-duction 10- to 100-fold by use of a helper phage lacking the kil gene and by UV irradiation of the phage stocks. I have also shown that mini-Mu-duction is a reliable complementation assay in rec+ as well as recA recipient strains. This genetic complementation test does not require prior gene localization and (due to the extended host range of phage Mu) should be applicable to many enterobacterial species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., Harshey R. M., Sarvetnick N., Bukhari A. I. Mechanism of bacteriophage Mu DNA transposition. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):311–322. doi: 10.1101/sqb.1981.045.01.043. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Littel K. J., Jackowski S. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1982 Mar;149(3):916–922. doi: 10.1128/jb.149.3.916-922.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet L., Faelen M., Lefèbvre N., Résibois A., Toussaint A., van Gijsegem F. Genetic study of Mu transposition and Mu-mediated chromosomal rearrangements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):355–363. doi: 10.1101/sqb.1981.045.01.049. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A., Resibois A. Mini-muduction: a new mode of gene transfer mediated by mini-mu. Mol Gen Genet. 1979 Oct 3;176(2):191–197. doi: 10.1007/BF00273213. [DOI] [PubMed] [Google Scholar]
- Gardner J. F., Smith O. H. Operator-promoter functions in the threonine operon of Escherichia coli. J Bacteriol. 1975 Oct;124(1):161–166. doi: 10.1128/jb.124.1.161-166.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M., Bukhari A. I. Heterogeneous host DNA attached to the left end of mature bacteriophage Mu DNA. Nature. 1981 Jul 9;292(5819):175–176. doi: 10.1038/292175a0. [DOI] [PubMed] [Google Scholar]
- Golub E. I., Low K. B. Indirect stimulation of genetic recombination. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1401–1405. doi: 10.1073/pnas.80.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda D. K., Radding C. M. By searching processively RecA protein pairs DNA molecules that share a limited stretch of homology. Cell. 1983 Sep;34(2):647–654. doi: 10.1016/0092-8674(83)90397-5. [DOI] [PubMed] [Google Scholar]
- Goosen T., Giphart-Gassler M., Van de Putte P. Bacteriophage Mu DNA replication is stimulated by non-essential early functions. Mol Gen Genet. 1982;186(1):135–139. doi: 10.1007/BF00422925. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Transduction by bacteriophage MU-1. Virology. 1973 Sep;55(1):103–117. doi: 10.1016/s0042-6822(73)81012-8. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill G. S., Curtiss R., 3rd Genetic characterization of Mu-like bacteriophage D108. J Virol. 1978 Sep;27(3):513–518. doi: 10.1128/jvi.27.3.513-518.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon H., Bukhari A. I. DNA rearrangements associated with reversion of bacteriophage Mu-induced mutations. Genetics. 1981 May;98(1):1–24. doi: 10.1093/genetics/98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèbvre N., Toussaint A. Transfer of Salmonella typhimurium and Klebsiella pneumoniae genes in E. coli K12 by mini-muduction. Mol Gen Genet. 1981;181(2):268–272. doi: 10.1007/BF00268436. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Welliver R. A., Witkowski T. A. Specialized transduction with lambda plac5: dependence on recB. J Bacteriol. 1982 Jun;150(3):1485–1488. doi: 10.1128/jb.150.3.1485-1488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. E., Felton J., Wright A. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature. 1981 Oct 22;293(5834):625–629. doi: 10.1038/293625a0. [DOI] [PubMed] [Google Scholar]
- Résibois A., Toussaint A., van Gijsegem F., Faelen M. Physical characterization of mini-mu and mini-D108. Gene. 1981 Jun-Jul;14(1-2):103–113. doi: 10.1016/0378-1119(81)90152-9. [DOI] [PubMed] [Google Scholar]
- Schaus N. A., Wright A. Inhibition of Escherichia coli exonuclease V by bacteriophage Mu. Virology. 1980 Apr 15;102(1):214–217. doi: 10.1016/0042-6822(80)90083-5. [DOI] [PubMed] [Google Scholar]
- Schultz D. W., Taylor A. F., Smith G. R. Escherichia coli RecBC pseudorevertants lacking chi recombinational hotspot activity. J Bacteriol. 1983 Aug;155(2):664–680. doi: 10.1128/jb.155.2.664-680.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]



