Abstract
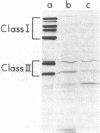
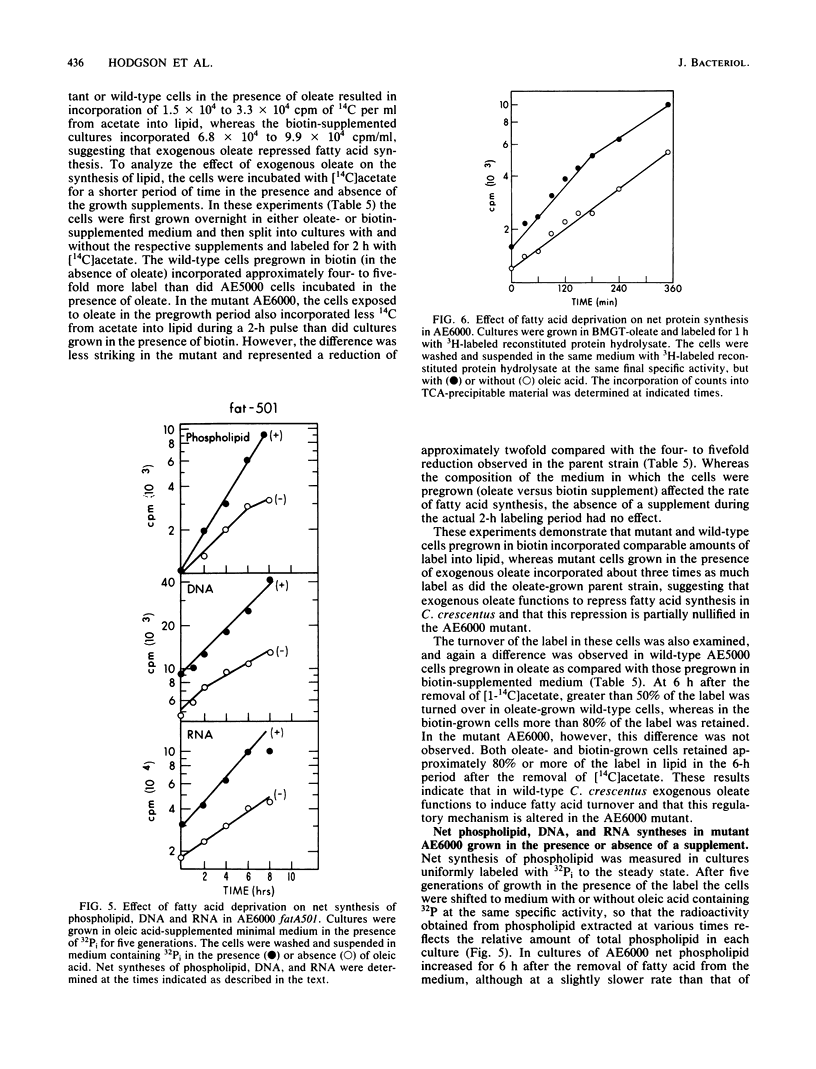
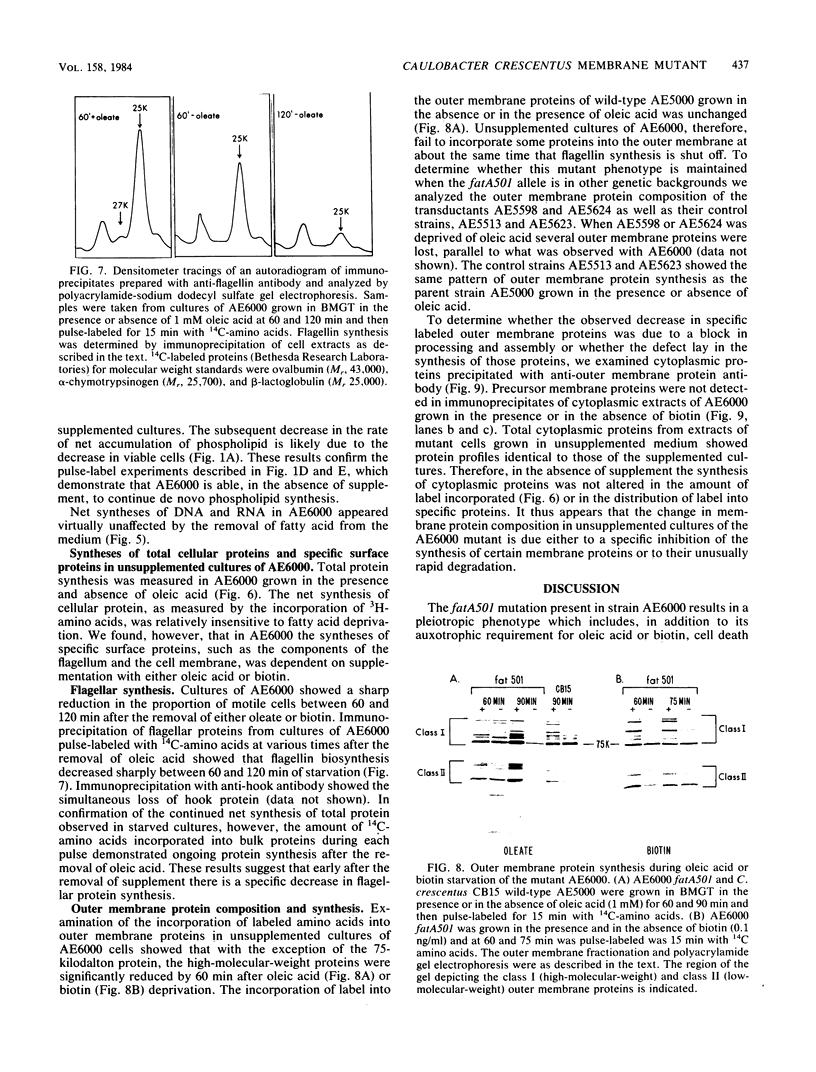
A mutant of Caulobacter crescentus has been isolated which has an auxotrophic requirement for unsaturated fatty acids or biotin for growth on medium containing glucose as the carbon source. This mutant exhibits a pleiotropic phenotype which includes (i) the auxotrophic requirement, (ii) cell death in cultures attempting to grow on glucose in the absence of fatty acids or biotin, and (iii) a major change in the outer membrane protein composition before cell death. This genetic lesion did not appear to affect directly a fatty acid biosynthetic reaction because fatty acid and phospholipid syntheses were found to continue in the absence of supplement. Oleic acid repressed fatty acid biosynthesis and induced fatty acid degradation in the wild-type parent, AE5000 . The mutant strain, AE6000 , was altered in both of these regulatory functions. The AE6000 mutant also showed specific inhibition of the synthesis of outer membrane and flagellar proteins. Total phospholipid, DNA, RNA, and protein syntheses were unaffected. The multiple phenotypes of the AE6000 mutant were found to cosegregate and to map between hclA and lacA on the C. crescentus chromosome. The defect in this mutant appears to be associated with a regulatory function in membrane biogenesis and provides evidence for a direct coordination of membrane protein synthesis and lipid metabolism in C. crescentus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N., Evinger M., Parker G. Generation of asymmetry during development. Segregation of type-specific proteins in Caulobacter. J Cell Biol. 1979 Apr;81(1):123–136. doi: 10.1083/jcb.81.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
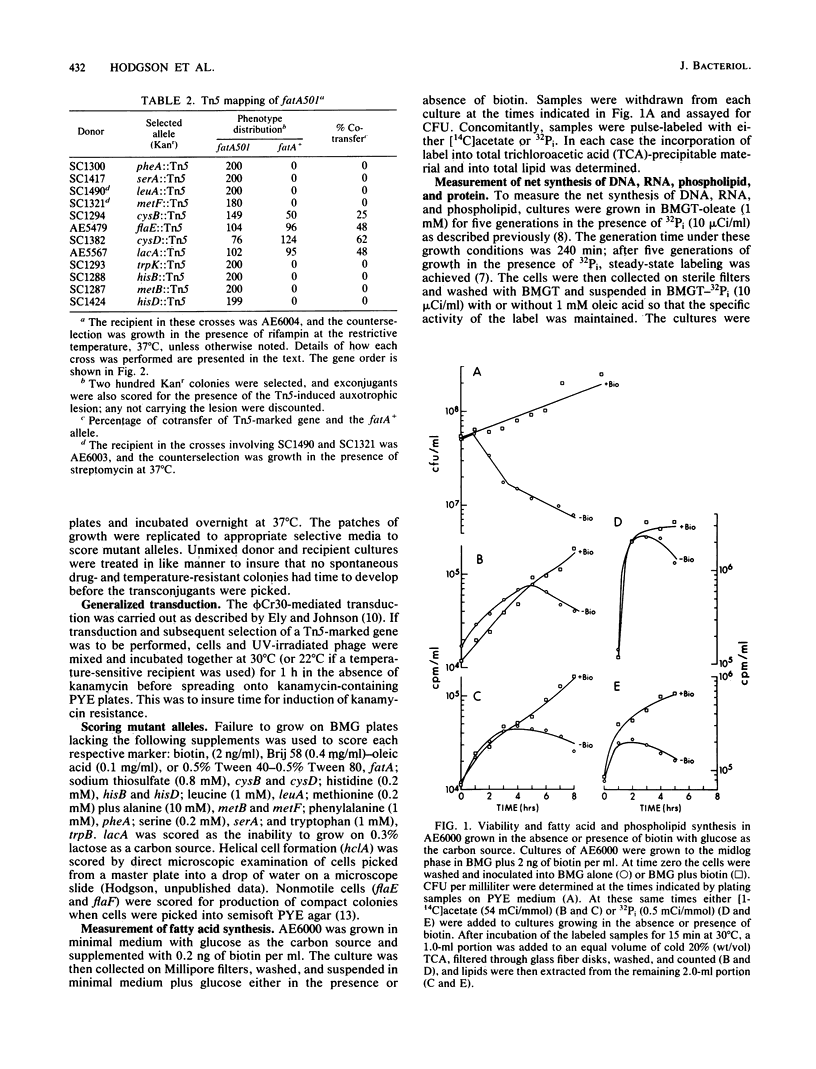
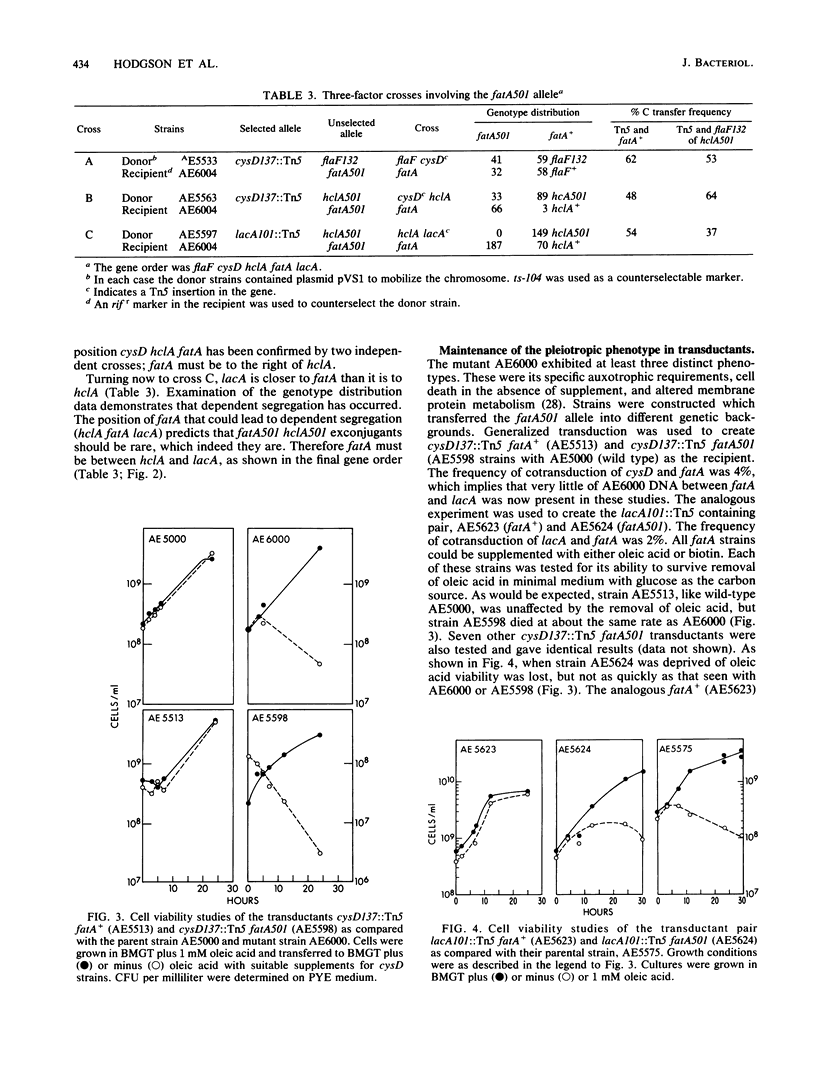
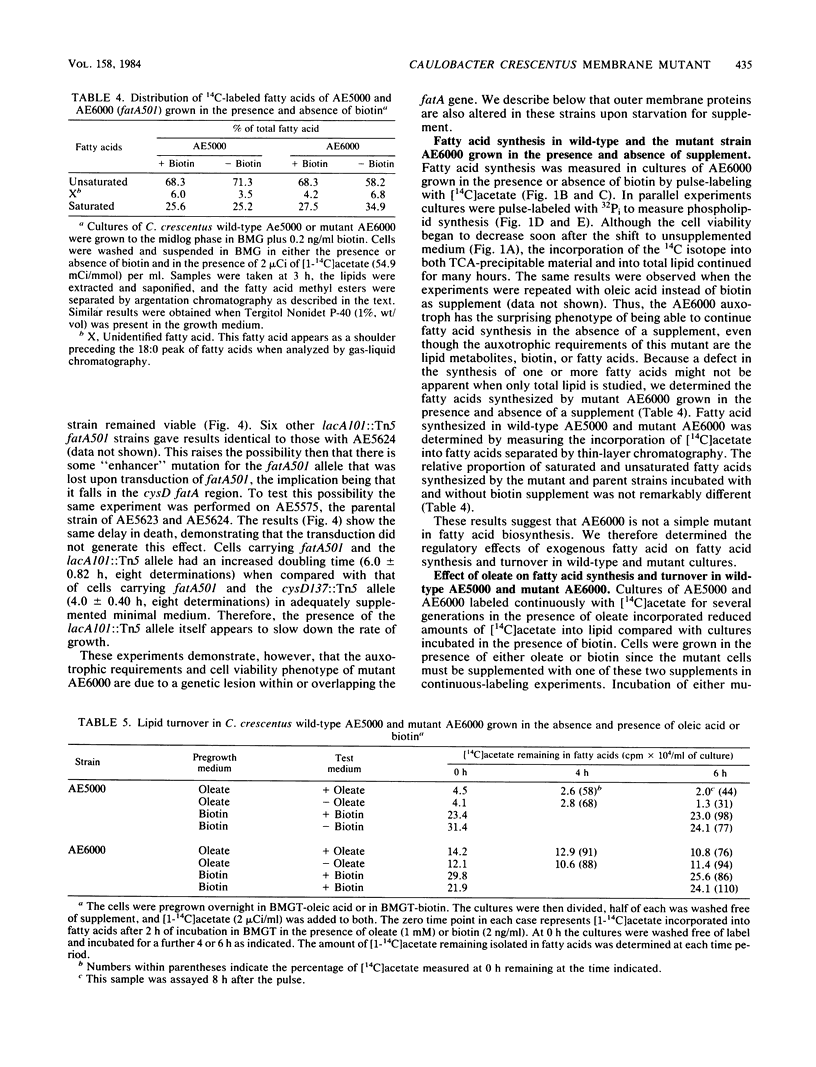
- Barrett J. T., Croft R. H., Ferber D. M., Gerardot C. J., Schoenlein P. V., Ely B. Genetic mapping with Tn5-derived auxotrophs of Caulobacter crescentus. J Bacteriol. 1982 Aug;151(2):888–898. doi: 10.1128/jb.151.2.888-898.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. T., Rhodes C. S., Ferber D. M., Jenkins B., Kuhl S. A., Ely B. Construction of a genetic map for Caulobacter crescentus. J Bacteriol. 1982 Mar;149(3):889–896. doi: 10.1128/jb.149.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Bender R. A., Mansour J., Henry S., Shapiro L. Caulobacter cresentus mutant defective in membrane phospholipid synthesis. J Bacteriol. 1979 Nov;140(2):612–619. doi: 10.1128/jb.140.2.612-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Shapiro L., Henry S. Membrane phospholipid composition of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1130–1136. doi: 10.1128/jb.135.3.1130-1136.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Weissborn A., Amemiya K., Mansour J., Henry S., Shapiro L., Bender R. The effect of termination of membrane phospholipid synthesis on cell-dependent events in Caulobacter. J Mol Biol. 1980 Apr;138(2):401–409. doi: 10.1016/0022-2836(80)90295-8. [DOI] [PubMed] [Google Scholar]
- Ely B., Johnson R. C. Generalized Transduction in CAULOBACTER CRESCENTUS. Genetics. 1977 Nov;87(3):391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Transfer of drug resistance factors to the dimorphic bacterium Caulobacter crescentus. Genetics. 1979 Mar;91(3):371–380. doi: 10.1093/genetics/91.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts V., Shaw P., Shapiro L., Henry S. Synthesis and utilization of fatty acids by wild-type and fatty acid auxotrophs of Caulobacter crescentus. J Bacteriol. 1982 Sep;151(3):1269–1278. doi: 10.1128/jb.151.3.1269-1278.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Bohlander M., Nunn W. D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol. 1980 Aug;143(2):720–725. doi: 10.1128/jb.143.2.720-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour J. D., Henry S., Shapiro L. Differential membrane phospholipid synthesis during the cell cycle of Caulobacter crescentus. J Bacteriol. 1980 Jan;141(1):262–269. doi: 10.1128/jb.141.1.262-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour J. D., Henry S., Shapiro L. Phospholipid biosynthesis is required for stalk elongation in Caulobacter crescentus. J Bacteriol. 1981 Mar;145(3):1404–1409. doi: 10.1128/jb.145.3.1404-1409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Giffin K., Clark D., Cronan J. E., Jr Role for fadR in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1983 May;154(2):554–560. doi: 10.1128/jb.154.2.554-560.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Mansour J., Shaw P., Henry S. Synthesis of specific membrane proteins is a function of DNA replication an phospholipid synthesis in Caulobacter crescentus. J Mol Biol. 1982 Aug 5;159(2):303–322. doi: 10.1016/0022-2836(82)90497-1. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Cohen M., Harder M. E. The effect of exogenous fatty acids on fatty acid metabolism in Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1699–1707. [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]