Abstract
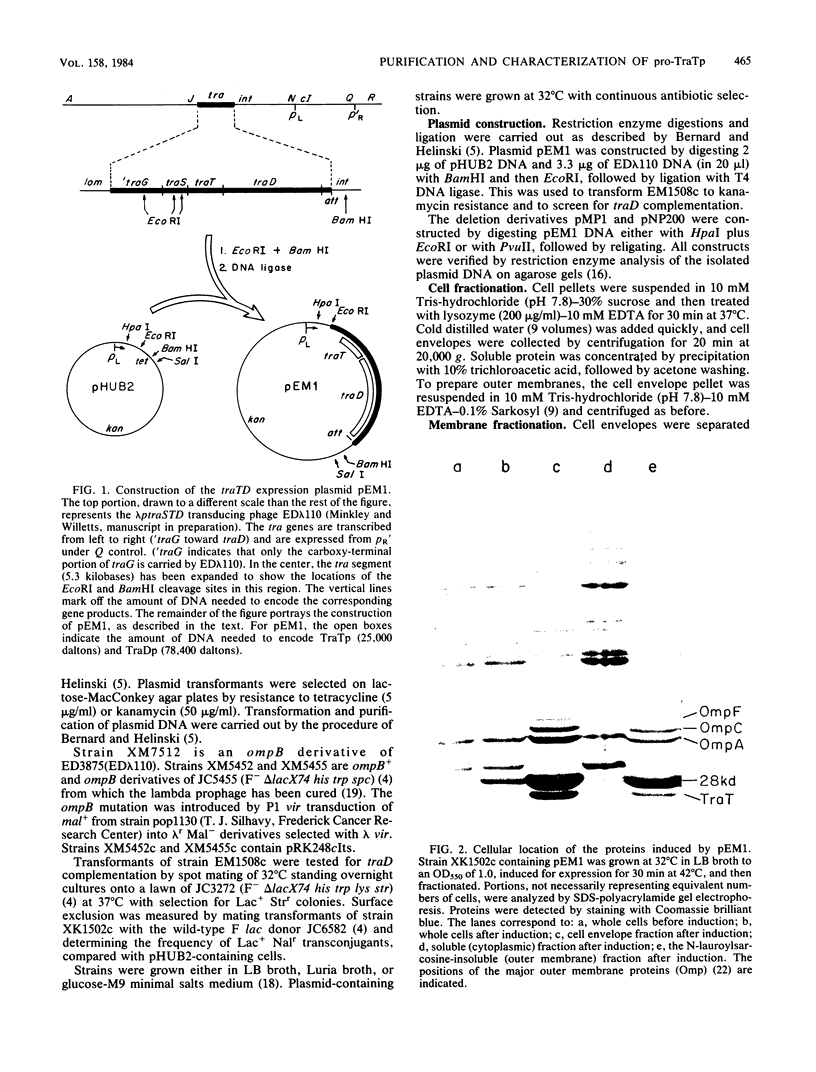
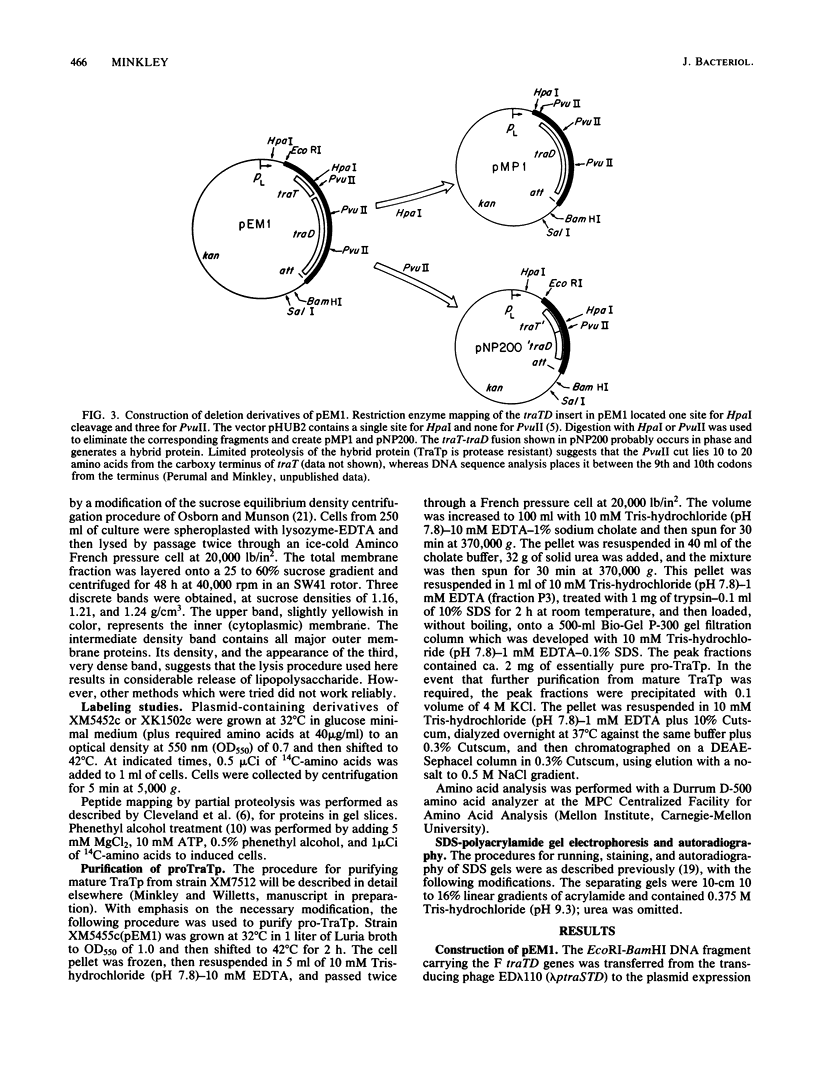
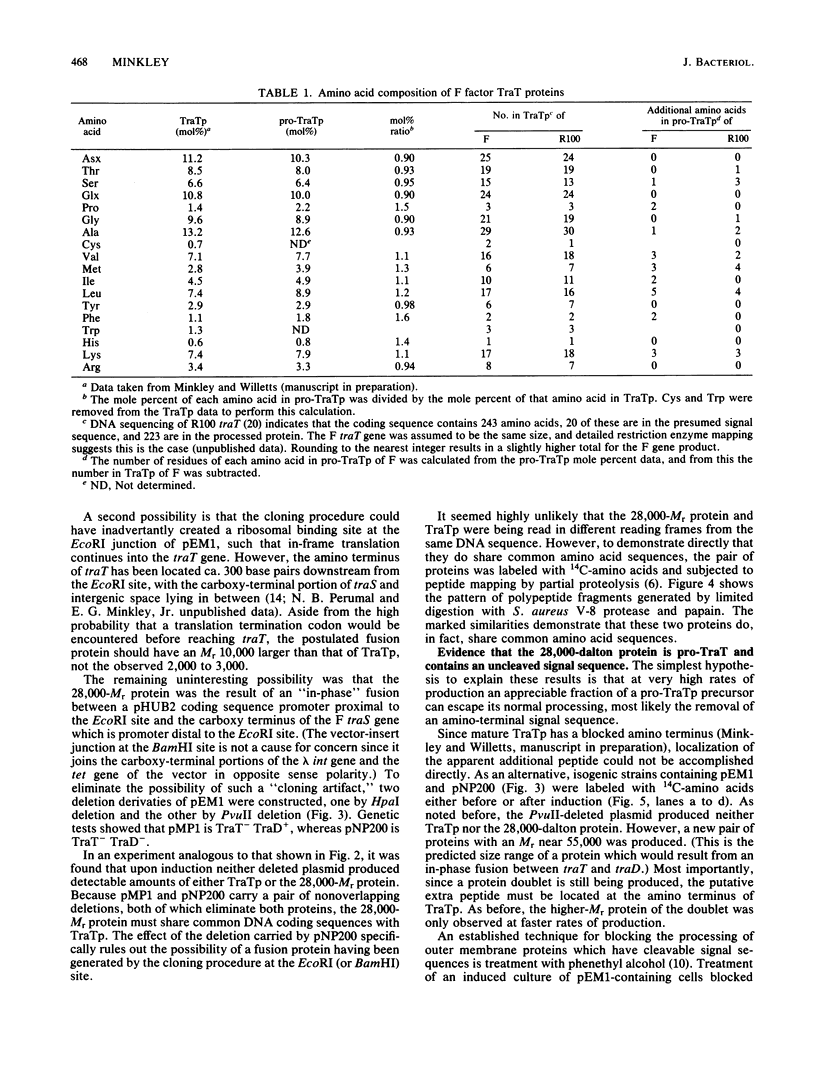
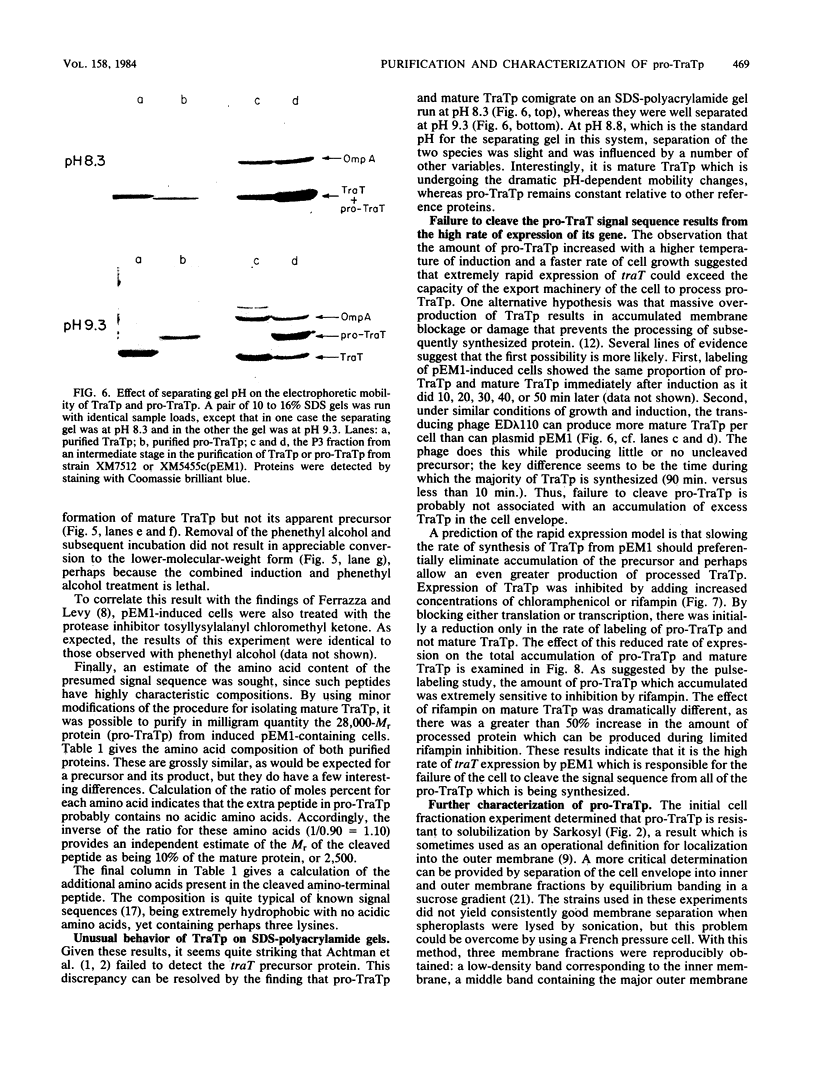
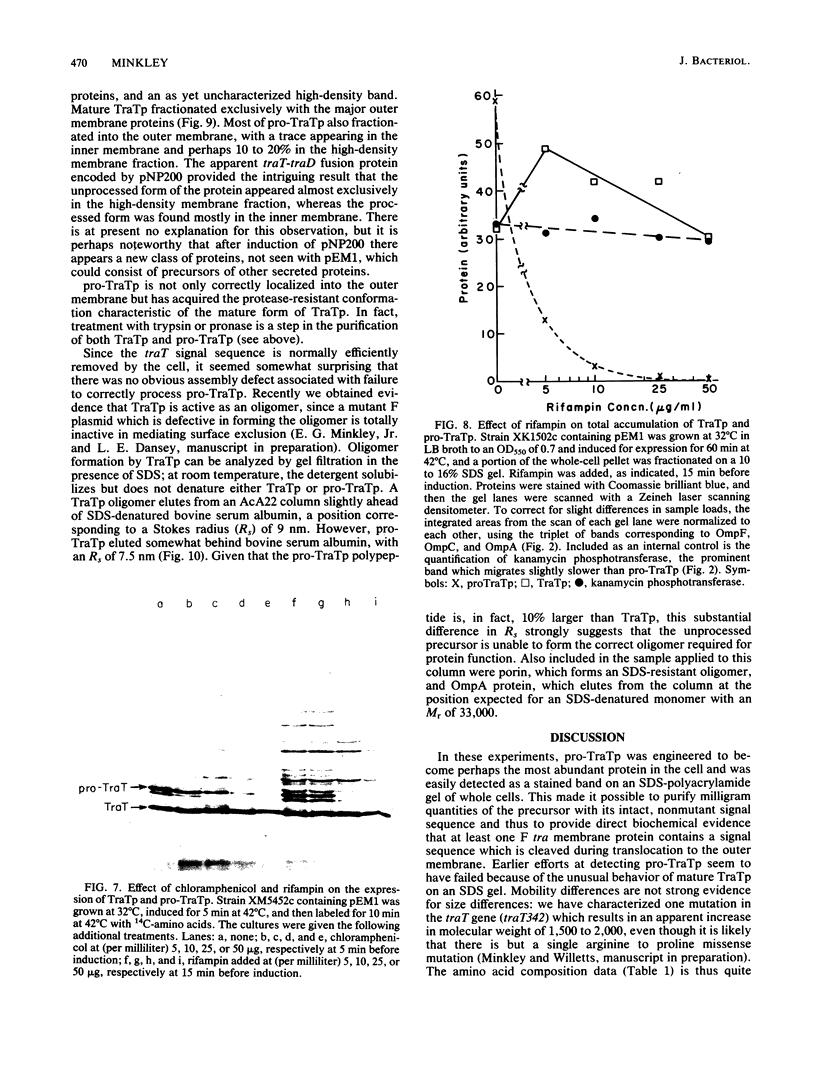
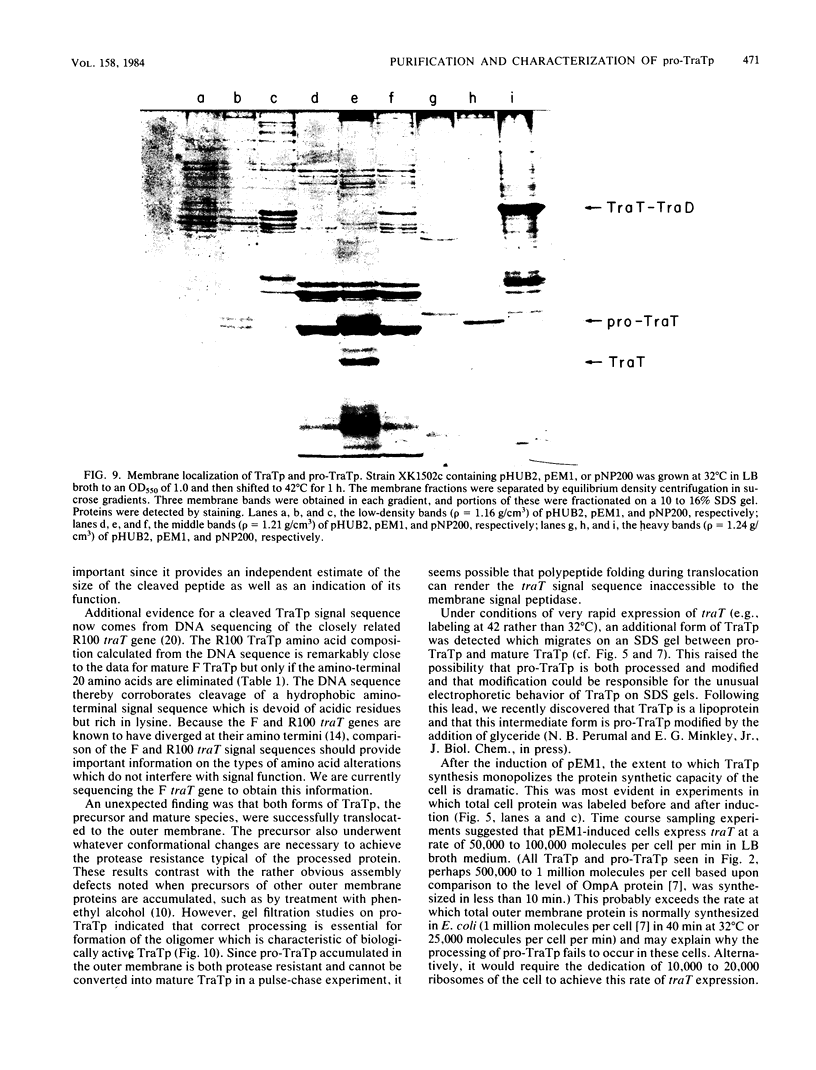
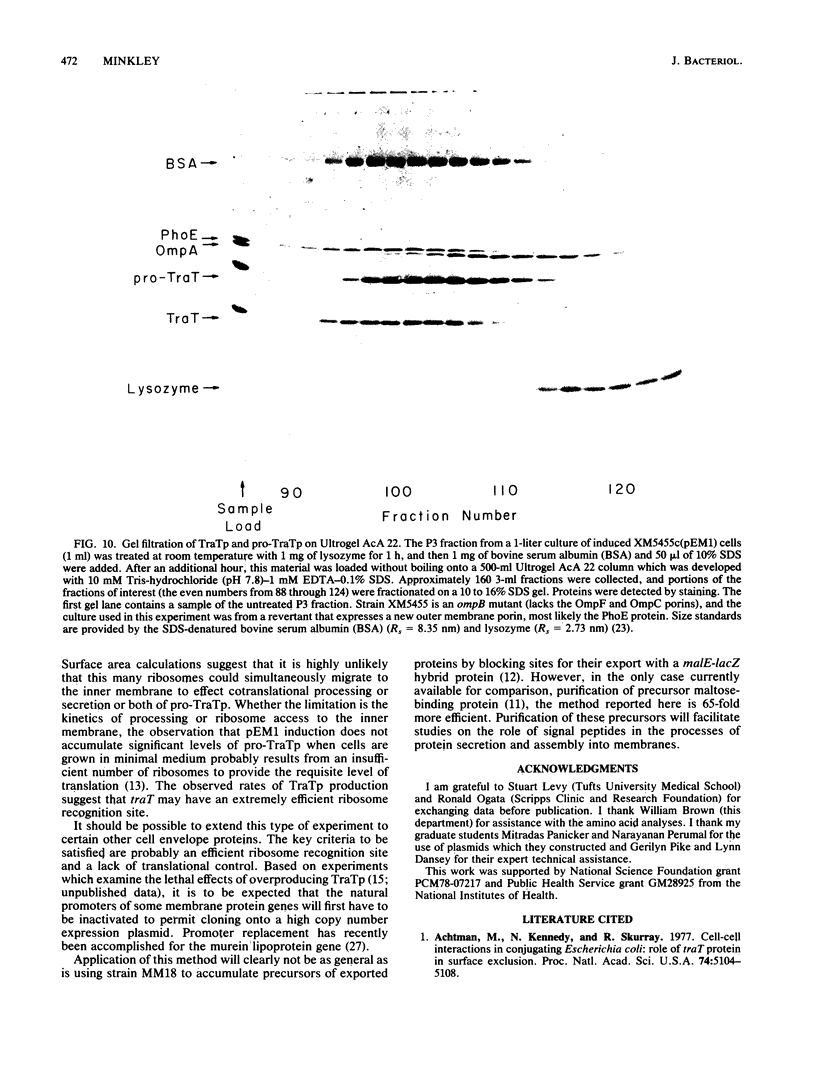
The structural gene for the F sex factor outer membrane surface exclusion protein ( traT ) was cloned onto a high-copy-number plasmid where it is expressed from the phage lambda promoter pL. Conditional control over expression was provided by a temperature-sensitive lambda cI repressor. Induction of pL produced large quantities of the traT gene product ( TraTp ) and, in rich growth media, even larger amounts of a higher-molecular-weight form of TraTp . This polypeptide was purified and characterized as a pro- TraTp precursor, which contains at its amino terminus a typical signal-like sequence, which is not present in the mature form of TraTp as isolated from the outer membrane of F-containing cells. Accumulation of pro- TraTp seemed not to result from the jamming of export sites, as in another system for obtaining precursors of secreted proteins, but rather from overwhelming kinetically the ability of the cell to process exported proteins. Although pro- TraTp appeared to be successfully translocated to the outer membrane, it was defective in forming the oligomeric structure required for surface exclusion function. The procedure used is not a general method but can be applied to certain other secreted proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Kusecek B., Schwuchow S., Willetts N. A genetic analysis of F sex factor cistrons needed for surface exclusion in Escherichia coli. J Mol Biol. 1980 Apr 25;138(4):779–795. doi: 10.1016/0022-2836(80)90065-0. [DOI] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biosynthesis of a plasmid-encoded outer membrane surface exclusion protein involves processing from a precursor polypeptide. J Biol Chem. 1980 Oct 10;255(19):8955–8958. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Ito K. Purification of the precursor form of maltose-binding protein, a periplasmic protein of Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):9895–9897. [PubMed] [Google Scholar]
- Manning P. A., Morelli G. DNA homology of the promoter-distal regions of the tra operons of sex factors F and R100 in Escherichia coli K-12. J Bacteriol. 1982 Apr;150(1):389–394. doi: 10.1128/jb.150.1.389-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Timmis J. K., Moll A., Timmis K. N. Mutants that overproduce TraTp, a plasmid-specified major outer membrane protein of Escherichia coli. Mol Gen Genet. 1982;187(3):426–431. doi: 10.1007/BF00332623. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Ippen-Ihler K. Identification of a membrane protein associated with expression of the surface exclusion region of the F transfer operon. J Bacteriol. 1977 Mar;129(3):1613–1622. doi: 10.1128/jb.129.3.1613-1622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Winters C., Levine R. P. Nucleotide sequence analysis of the complement resistance gene from plasmid R100. J Bacteriol. 1982 Aug;151(2):819–827. doi: 10.1128/jb.151.2.819-827.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Ippolito C., Inukai M., Inouye M. Temperature-sensitive processing of outer membrane lipoprotein in an Escherichia coli mutant. J Bacteriol. 1982 Dec;152(3):1163–1168. doi: 10.1128/jb.152.3.1163-1168.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Date T., Wickner W. Leader peptidase is found in both the inner and outer membranes of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3593–3597. [PubMed] [Google Scholar]









