Abstract
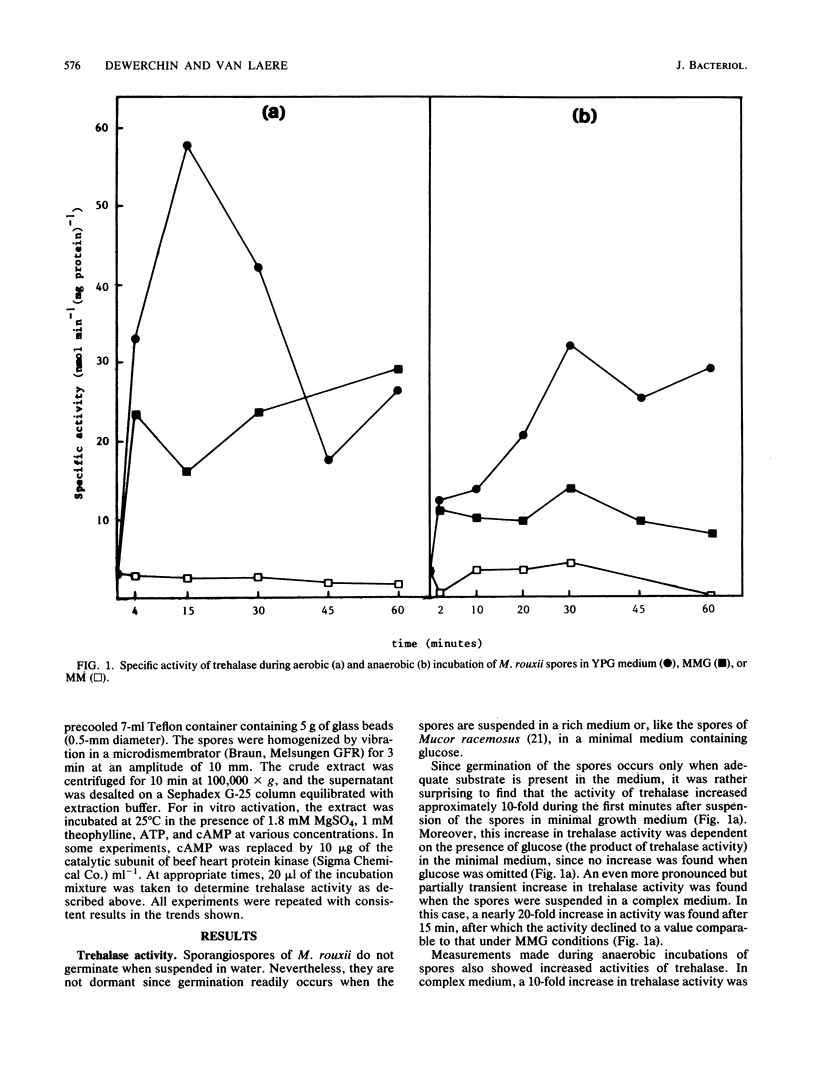
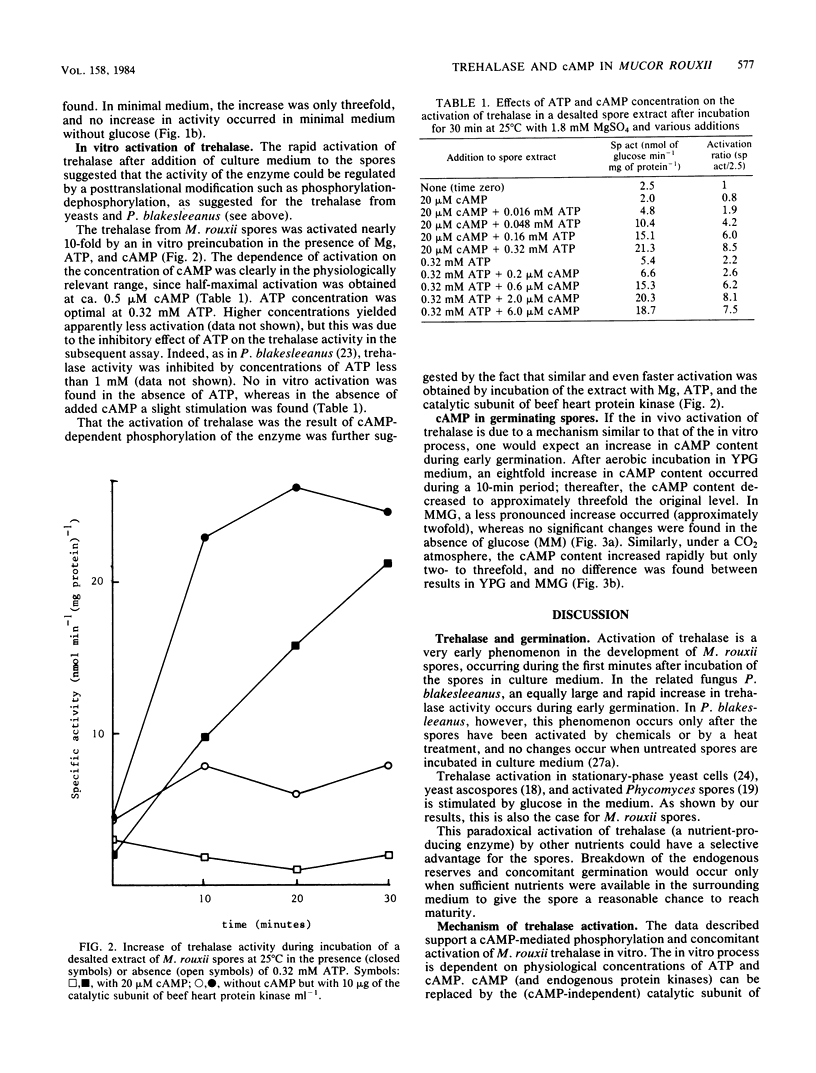
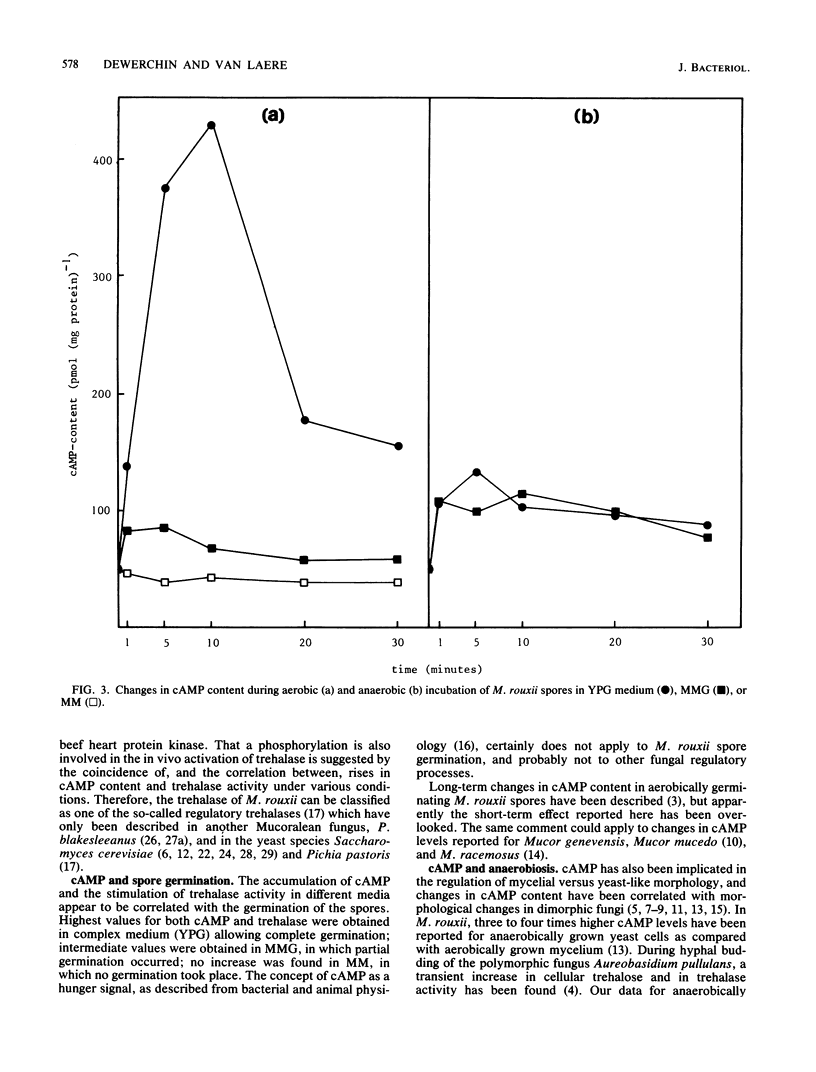
Incubation of Mucor rouxii sporangiospores in complex medium under aerobic conditions resulted in a transient 20-fold increase in trehalase activity. Maximum activity was reached after 15 min. Simultaneously, the cyclic AMP (cAMP) content increased approximately eightfold, reaching a maximum within 10 min. Increases in trehalase activity and cAMP content were also observed under anaerobic conditions (CO2). The extent of trehalase activation and the changes in cAMP content, during both aerobic and anaerobic incubation, varied with the medium used. Trehalase was activated in vitro by a cAMP- and ATP-dependent process. An even faster activation was obtained when cAMP was replaced by the catalytic subunit of beef heart protein kinase. The coincidence of, and the correlation between, increased cAMP contents and trehalase activities support the involvement of a cAMP-dependent phosphorylation in the in vivo regulation of trehalase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantore M. L., Galvagno M. A., Passeron S. Variations in the levels of cyclic adenosine 3':5'-monophosphate and in the activities of adenylate cyclase and cyclic adenosine 3':5'-monophosphate phosphodiesterase during aerobic morphogenesis of Mucor rouxii. Arch Biochem Biophys. 1980 Feb;199(2):312–320. doi: 10.1016/0003-9861(80)90286-6. [DOI] [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londesborough J. Cyclic nucleotide-dependent inactivation of yeast fructose 1,6-bisphosphatase by ATP. FEBS Lett. 1982 Aug 2;144(2):269–272. doi: 10.1016/0014-5793(82)80652-2. [DOI] [PubMed] [Google Scholar]
- Maresca B., Medoff G., Schlessinger D., Kobayashi G. S. Regulation of dimorphism in the pathogenic fungus Histoplasma capsulatum. Nature. 1977 Mar 31;266(5601):447–448. doi: 10.1038/266447a0. [DOI] [PubMed] [Google Scholar]
- Medoff J., Jacobson E., Medoff G. Regulation of dimorphism in Histoplasma capsulatum by cyclic adenosine 3',5'-monophosphate. J Bacteriol. 1981 Mar;145(3):1452–1455. doi: 10.1128/jb.145.3.1452-1455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi M., Niimi K., Tokunaga J., Nakayama H. Changes in cyclic nucleotide levels and dimorphic transition in Candida albicans. J Bacteriol. 1980 Jun;142(3):1010–1014. doi: 10.1128/jb.142.3.1010-1014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. Cyclic adenosine 3',5'-monophosphate and germination of sporangiospores from the fungus Mucor. Arch Microbiol. 1980 Jun;126(2):133–140. doi: 10.1007/BF00511218. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Ross J. F. Relationship of internal cyclic AMP levels, rates of protein synthesis and mucor dimorphism. Arch Microbiol. 1981 Jul;129(5):353–356. doi: 10.1007/BF00406461. [DOI] [PubMed] [Google Scholar]
- Ortiz C. H., Maia J. C., Tenan M. N., Braz-Padrão G. R., Mattoon J. R., Panek A. D. Regulation of yeast trehalase by a monocyclic, cyclic AMP-dependent phosphorylation-dephosphorylation cascade system. J Bacteriol. 1983 Feb;153(2):644–651. doi: 10.1128/jb.153.2.644-651.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paveto C., Epstein A., Passeron S. Studies on cyclic adenosine 3' ,5'-monophosphate levels, Adenylate cyclase and phosphodiesterase activities in the dimorphic fungus Mucor rouxii. Arch Biochem Biophys. 1975 Aug;169(2):449–457. doi: 10.1016/0003-9861(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Respiratory capacity, cyclic adenosine 3',5'-monophosphate, and morphogenesis of Mucor racemosus. J Bacteriol. 1975 Oct;124(1):134–139. doi: 10.1128/jb.124.1.134-139.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco M., Maresca B., Kumar B. V., Kobayashi G. S., Medoff G. Temperature- and cyclic nucleotide-induced phase transitions of Histoplasma capsulatum. J Bacteriol. 1981 Apr;146(1):117–120. doi: 10.1128/jb.146.1.117-120.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., den Hollander J. A., Shulman R. G. Changes in the activity and properties of trehalase during early germination of yeast ascospores: correlation with trehalose breakdown as studied by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3503–3507. doi: 10.1073/pnas.79.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Tripp M. L., Paznokas J. L. Glucose-initiated germination of mucor racemosus sporangiospores. J Gen Microbiol. 1982 Mar;128(3):477–483. doi: 10.1099/00221287-128-3-477. [DOI] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Genetic and biochemical evidence that trehalase is a substrate of cAMP-dependent protein kinase in yeast. J Biol Chem. 1983 Sep 25;258(18):10867–10872. [PubMed] [Google Scholar]
- Van Assche J. A., Carlier A. R. Some properties of trehalase from Phycomyces blakesleeanus. Biochim Biophys Acta. 1975 May 23;391(1):154–161. doi: 10.1016/0005-2744(75)90161-8. [DOI] [PubMed] [Google Scholar]
- Van Laere A., Van Schaftingen E., Hers H. G. Fructose 2,6-bisphosphate and germination of fungal spores. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6601–6605. doi: 10.1073/pnas.80.21.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A., Schellenberg M. Does a cyclic AMP-dependent phosphorylation initiate the transfer of trehalase from the cytosol into the vacuoles in Saccharomyces cerevisiae? FEBS Lett. 1982 Dec 27;150(2):329–331. doi: 10.1016/0014-5793(82)80762-x. [DOI] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]


