Abstract
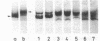
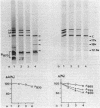
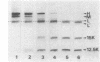
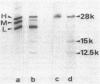
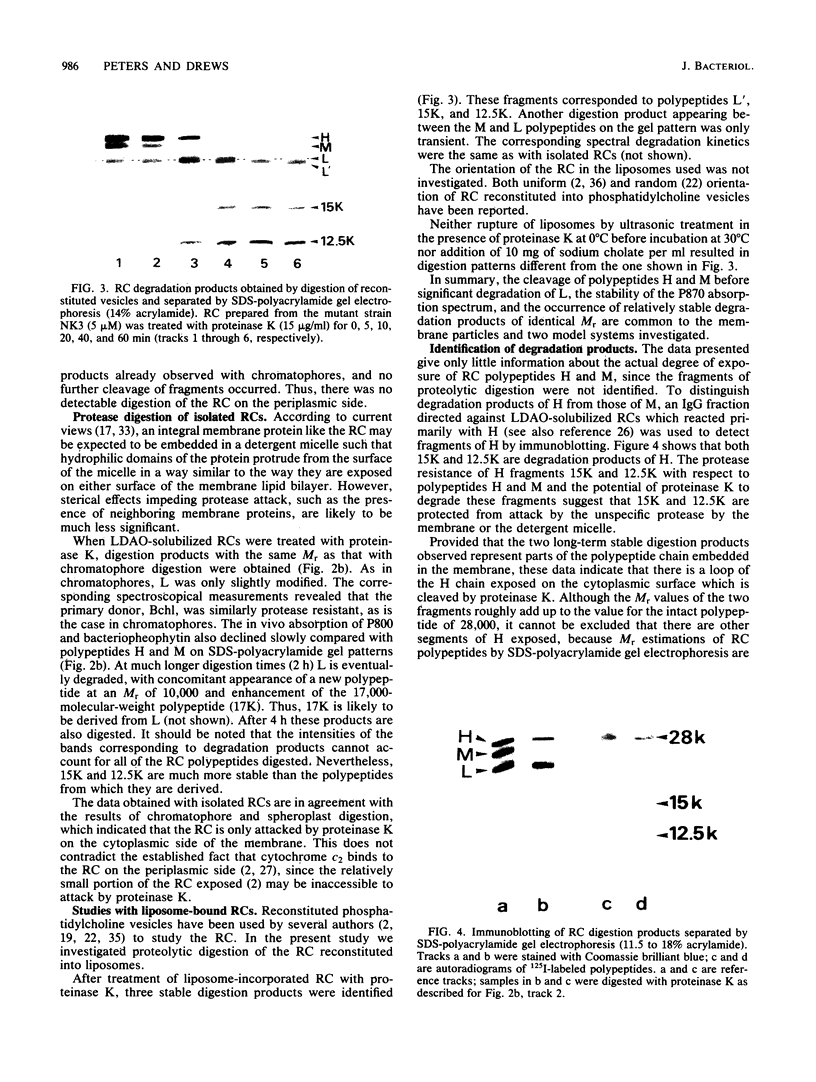
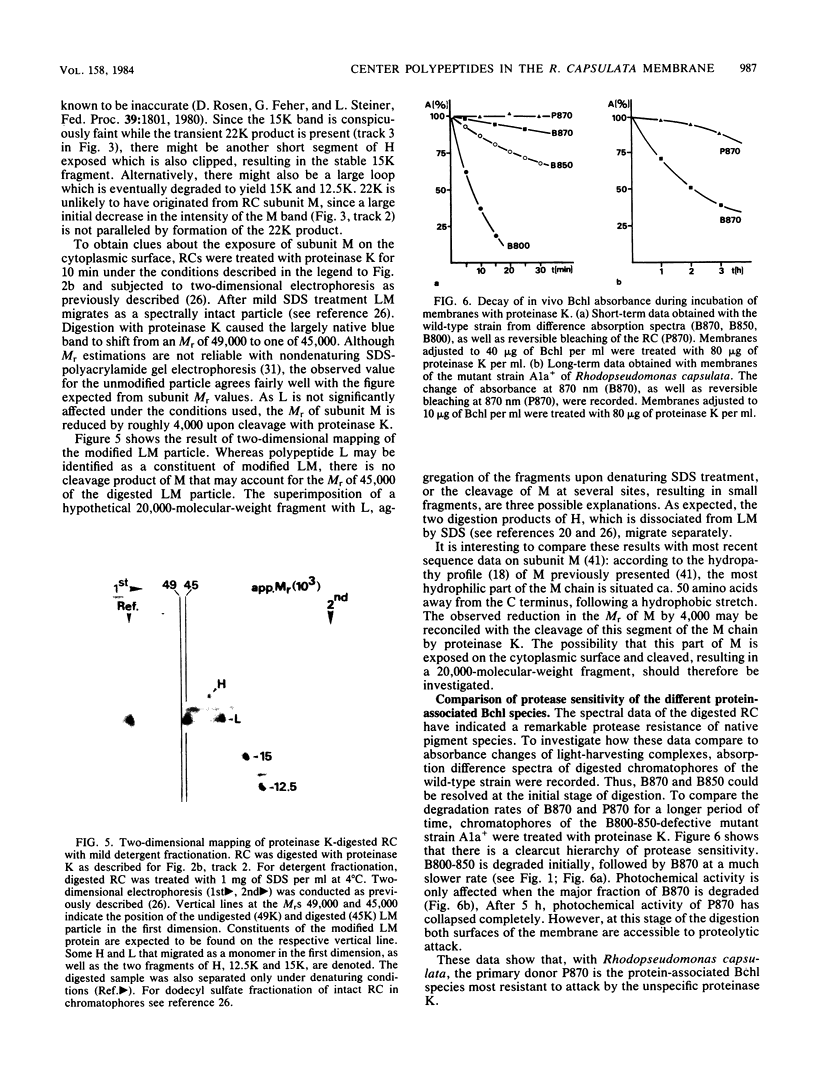
The exposure of the three polypeptide subunits H, M, and L of the photochemical reaction center (RC) on both surfaces of the membrane of Rhodopseudomonas capsulata was studied by partial proteolysis with proteinase K and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of of degradation products. The possible association of RC subunits with bacteriochlorophyll a and bacteriopheophytin was investigated by spectroscopical measurements. Chromatophores (inside-out oriented) and spheroplasts (right-side-out oriented), as well as purified, detergent-solubilized RCs and RCs reconstituted into phosphatidyl choline liposomes, were used. Subunit H of the RC was degraded to fragments with apparent MrS of 15,000 and 12,500, which were possibly derived from cleavage of a loop exposed on the cytoplasmic surface. Polypeptide M was digested at a comparable rate. The apparent Mr of M decreased by roughly 4,000 upon proteolytic cleavage. Subunit L was relatively insensitive to protease attack, except that a small peptide was clipped off. The primary donor P870 was also found to be only slightly affected proteinase K. All three RC subunits appear to be exposed on the chromatophore surface.
Full text
PDF

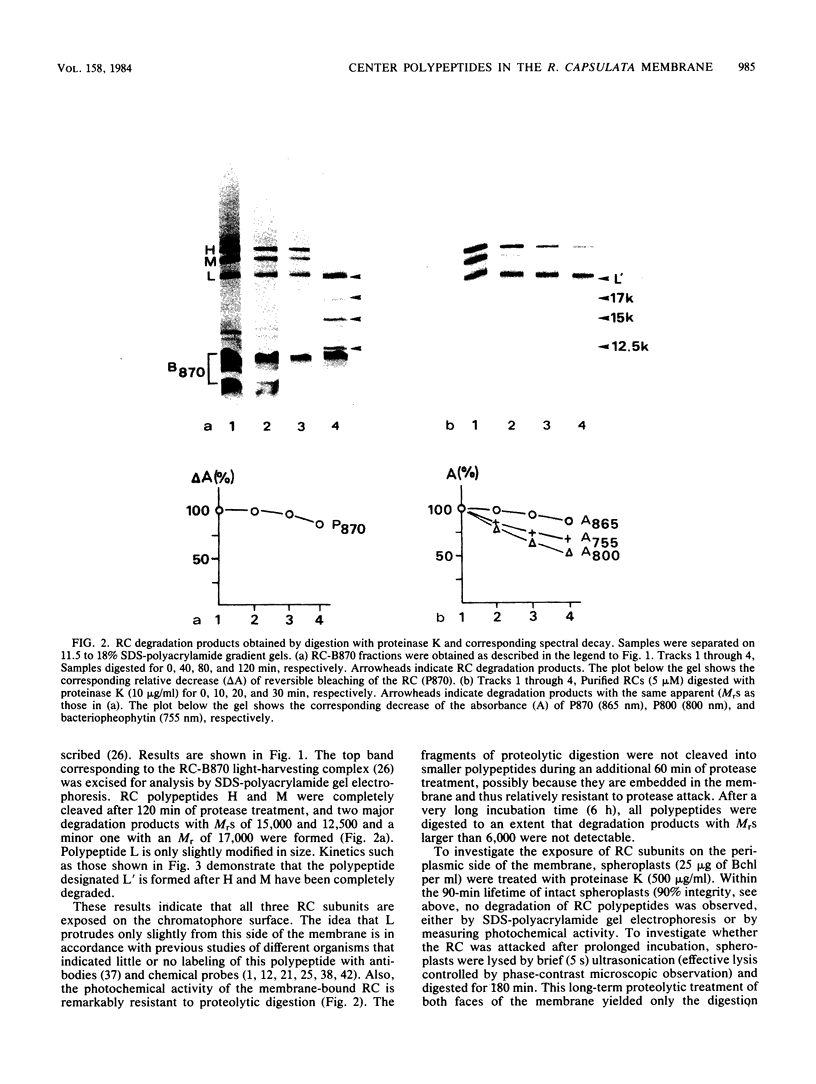


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann R. C., Gillies K., Takemoto J. Y. Membrane topography of the photosynthetic reaction center polypeptides of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Aug 4;20(16):4590–4596. doi: 10.1021/bi00519a012. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Mallon D. E., Niederman R. A. Assessment of Rhodopseudomonas sphaeroides chromatophore membrane asymmetry through bilateral antiserum adsorption studies. J Bacteriol. 1980 Jul;143(1):221–230. doi: 10.1128/jb.143.1.221-230.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G., Oelze J. Organization and differentiation of membranes of phototrophic bacteria. Adv Microb Physiol. 1981;22:1–92. doi: 10.1016/s0065-2911(08)60325-2. [DOI] [PubMed] [Google Scholar]
- Ebeling W., Hennrich N., Klockow M., Metz H., Orth H. D., Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974 Aug 15;47(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03671.x. [DOI] [PubMed] [Google Scholar]
- Feick R., Drews G. Isolation and characterization of light harvesting bacteriochlorophyll.protein complexes from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1978 Mar 13;501(3):499–513. doi: 10.1016/0005-2728(78)90117-2. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Francis G. A., Richards W. R. Localization of photosynthetic membrane components in Rhodopseudomonas sphaeroides by a radioactive labeling procedure. Biochemistry. 1980 Oct 28;19(22):5104–5111. doi: 10.1021/bi00563a026. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Nieth K. F., Drews G., Feick R. Photochemical reaction centers from Rhodopseudomonas capsulata. Arch Microbiol. 1975 Sep 30;105(1):43–45. doi: 10.1007/BF00447110. [DOI] [PubMed] [Google Scholar]
- Oelze J. Proteins exposed at the surface of chromatophores of Rhodospirillum rubrum: the orientation of isolated chromatophores. Biochim Biophys Acta. 1978 Jun 2;509(3):450–461. doi: 10.1016/0005-2736(78)90239-0. [DOI] [PubMed] [Google Scholar]
- Overfield R. E., Wraight C. A. Oxidation of cytochromes c and c2 by bacterial photosynthetic reaction centers in phospholipid vesicles. 1. Studies with neutral membranes. Biochemistry. 1980 Jul 8;19(14):3322–3327. doi: 10.1021/bi00555a034. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Dutton P. L., Blasie J. K. Structural studies on reconstituted reaction center-phosphatidylcholine membranes. Biochim Biophys Acta. 1979 Nov 8;548(2):348–373. doi: 10.1016/0005-2728(79)90141-5. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Dutton P. L., Blasie J. K. The reaction center profile structure derived from neutron diffraction. Biochim Biophys Acta. 1981 Apr 13;635(2):267–283. doi: 10.1016/0005-2728(81)90026-8. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Senior A. E. Secondary and tertiary structure of membrane proteins involved in proton translocation. Biochim Biophys Acta. 1983 Jul 15;726(2):81–95. doi: 10.1016/0304-4173(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Welte W., Hodapp N., Drews G. Studies on the size and composition of the isolated light-harvesting B800-850 pigment-protein complex of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1982 Feb;213(2):473–485. doi: 10.1016/0003-9861(82)90573-2. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G. D., Cogdell R. J., Lindsay J. G. Localization of the reaction-centre subunits in the intracytoplasmic membranes of Rhodopseudomonas sphaeroides and Rhodopseudomonas capsulata [proceedings]. Biochem Soc Trans. 1980 Apr;8(2):184–185. doi: 10.1042/bst0080184. [DOI] [PubMed] [Google Scholar]
- Werner S., Sebald W. Immunological techniques for studies on the biogenesis of mitochondrial membrane proteins. Methods Biochem Anal. 1981;27:109–170. doi: 10.1002/9780470110478.ch3. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürrer H., Snozzi M., Hanselmann K., Bachofen R. Localisation of the subunits of the photosynthetic reaction centers in the chromatophore membrane of Rhodospirillum rubrum. Biochim Biophys Acta. 1977 May 11;460(2):273–279. doi: 10.1016/0005-2728(77)90213-4. [DOI] [PubMed] [Google Scholar]