Abstract
Chromatium vinosum, an anaerobic photosynthetic purple sulfur bacterium, resembles aerobic bacterial cells in that it has an NADP-thioredoxin system composed of a single thioredoxin which is reduced by NADPH via NADP-thioredoxin reductase. Both protein components were purified to homogeneity, and some of their properties were determined. Chromatium vinosum thioredoxin was slightly larger than other bacterial thioredoxins (13 versus 12 kilodaltons) but was similar in its specificity (ability to activate chloroplast NADP-malate dehydrogenase more effectively than chloroplast fructose-1,6-bisphosphatase) and immunological properties. As in other bacteria, Chromatium vinosum NADP-thioredoxin reductase was an arsenite-sensitive flavoprotein composed of two 33.5-kilodalton subunits, that required thioredoxin for the NADPH-linked reduction of 5,5'-dithiobis(2-nitrobenzoic acid). Chromatium vinosum NADP-thioredoxin reductase very effectively reduced several different bacterial-type thioredoxins (Escherichia coli, Chlorobium thiosulfatophilum (this name has not been approved by the International Committee of Systematic Bacteriology), Rhizobium meliloti) but not others (Clostridium pasteurianum, spinach chloroplast thioredoxin m). The results show that Chromatium vinosum contains an NADP-thioredoxin system typical of evolutionarily more advanced microorganisms.
Full text
PDF
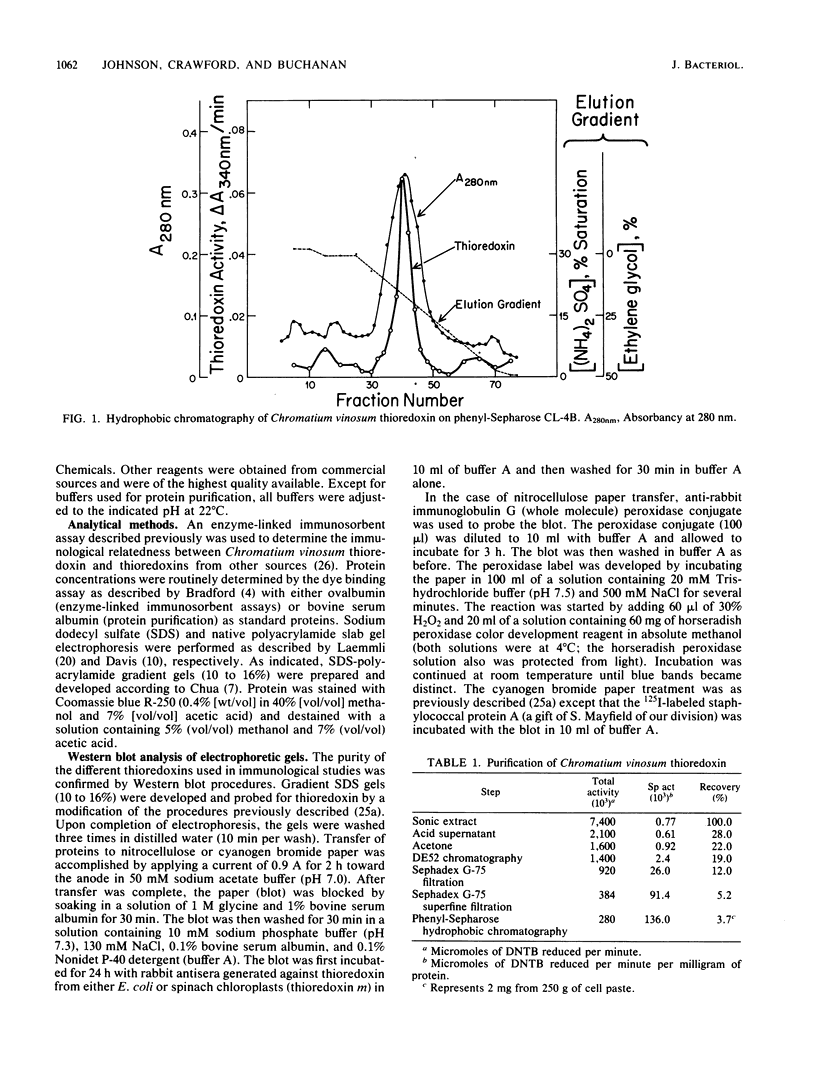

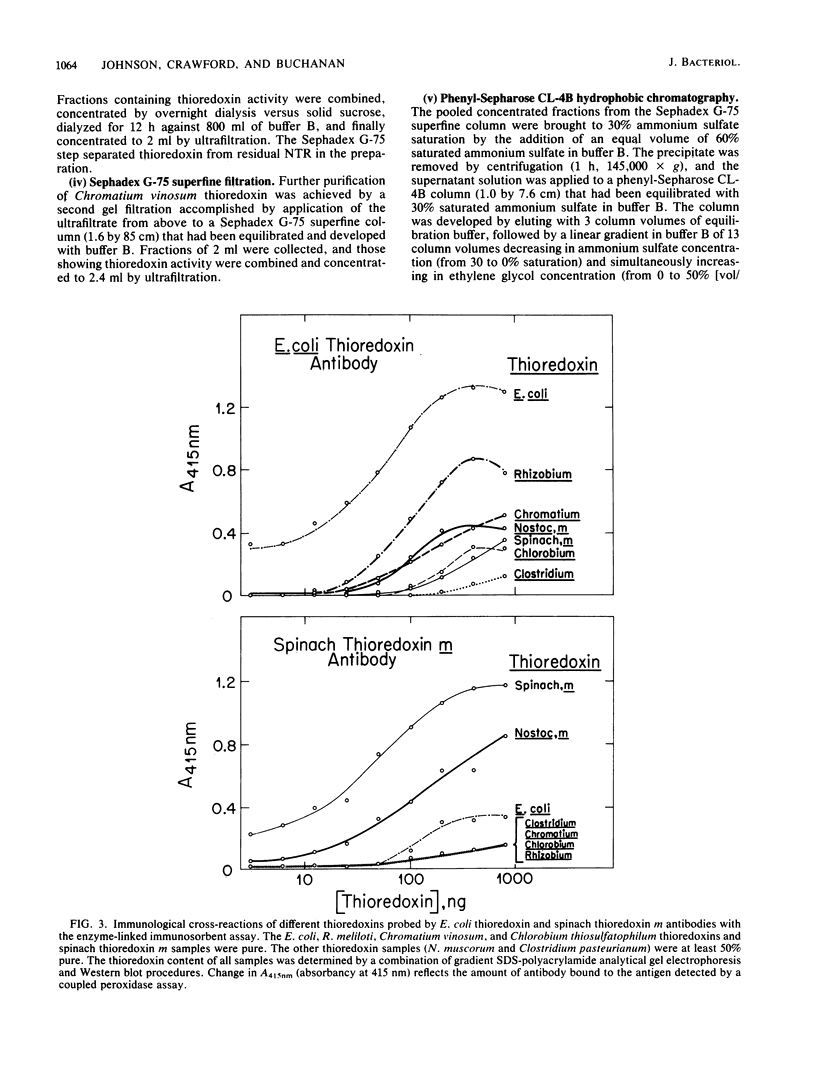

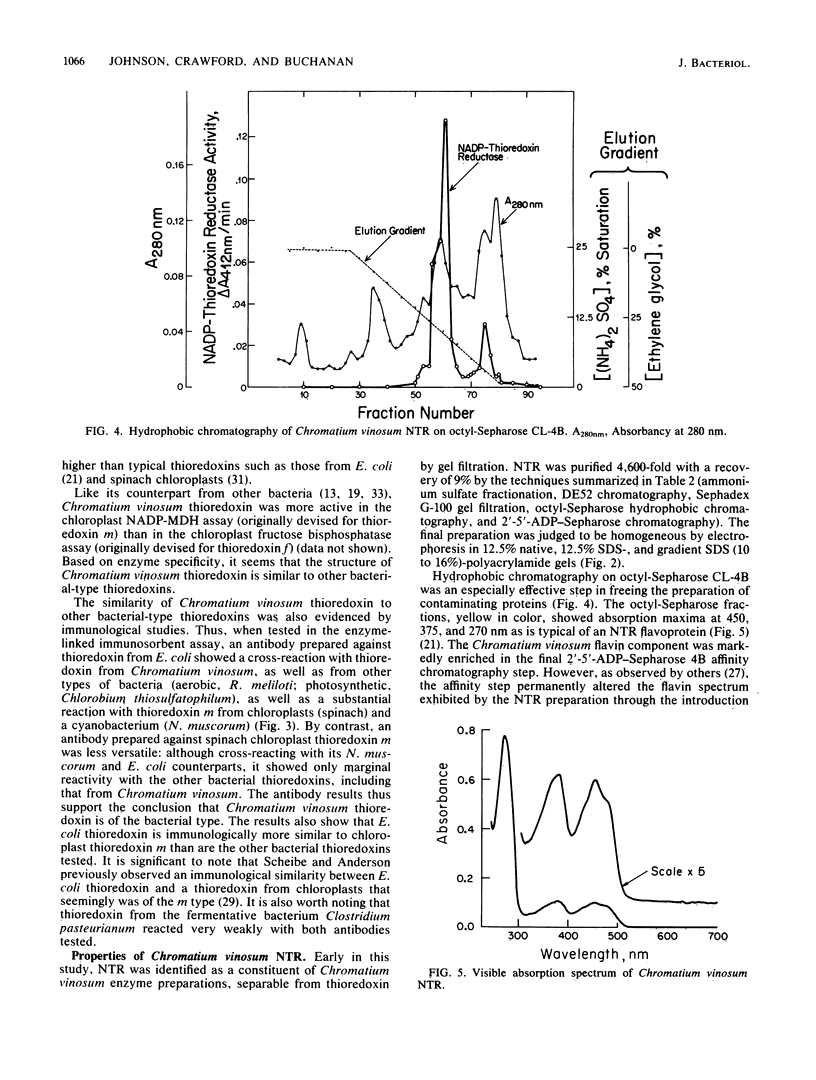


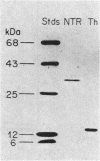
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachofen R., Arnon D. I. Crystalline ferredoxin from the photosynthetic bacterium Chromatium. Biochim Biophys Acta. 1966 Jun 8;120(2):259–265. doi: 10.1016/0926-6585(66)90345-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Wolosiuk R. A. Photosynthetic regulatory protein found in animal and bacterial cells. Nature. 1976 Dec 16;264(5587):669–670. doi: 10.1038/264669a0. [DOI] [PubMed] [Google Scholar]
- Clement-Metral J. D. Activation of ALA synthetase by reduced thioredoxin in Rhodopseudomonas spheroides Y. FEBS Lett. 1979 May 1;101(1):116–120. doi: 10.1016/0014-5793(79)81307-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Hall D. E., Baldesten A., Holmgren A., Reichard P. Yeast thioredoxin. Amino-acid sequence around the active-center disulfide of thioredoxin I and II. Eur J Biochem. 1971 Nov 11;23(2):328–335. doi: 10.1111/j.1432-1033.1971.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Cornwell K. L., Buchanan B. B. Ferredoxin/flavoprotein-linked pathway for the reduction of thioredoxin. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3681–3685. doi: 10.1073/pnas.80.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Jacquot J. P., Buchanan B. B. Enzyme Regulation in C(4) Photosynthesis : PURIFICATION AND PROPERTIES OF THIOREDOXIN-LINKED NADP-MALATE DEHYDROGENASE FROM CORN LEAVES. Plant Physiol. 1981 Aug;68(2):300–304. doi: 10.1104/pp.68.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot J. P., Maudinas B., Gadal P. Occurence of thioredoxin m activity in the photosynthetic bacteria Rhodopseudomonas capsulata. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1371–1376. doi: 10.1016/0006-291x(79)91218-x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P., THELANDER L. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES.V. PURIFICATION AND PROPERTIES OF THIOREDOXIN REDUCTASE FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3445–3452. [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Scheibe R., Anderson L. E. Dark modulation of NADP-dependent malate dehydrogenase and glucose-6-phosphate dehydrogenase in the chloroplast. Biochim Biophys Acta. 1981 Jun 12;636(1):58–64. doi: 10.1016/0005-2728(81)90075-x. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Maeda K., Tsugita A. Isomers in thioredoxins of spinach chloroplasts. Eur J Biochem. 1981 May;116(1):37–45. doi: 10.1111/j.1432-1033.1981.tb05297.x. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Maeda K., Schürmann P. Spinach chloroplast thioredoxins in evolutionary drift. Biochem Biophys Res Commun. 1983 Aug 30;115(1):1–7. doi: 10.1016/0006-291x(83)90960-9. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Wolosiuk R. A., Crawford N. A., Yee B. C., Buchanan B. B. Isolation of three thioredoxins from spinach leaves. J Biol Chem. 1979 Mar 10;254(5):1627–1632. [PubMed] [Google Scholar]