Abstract
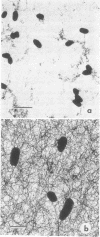
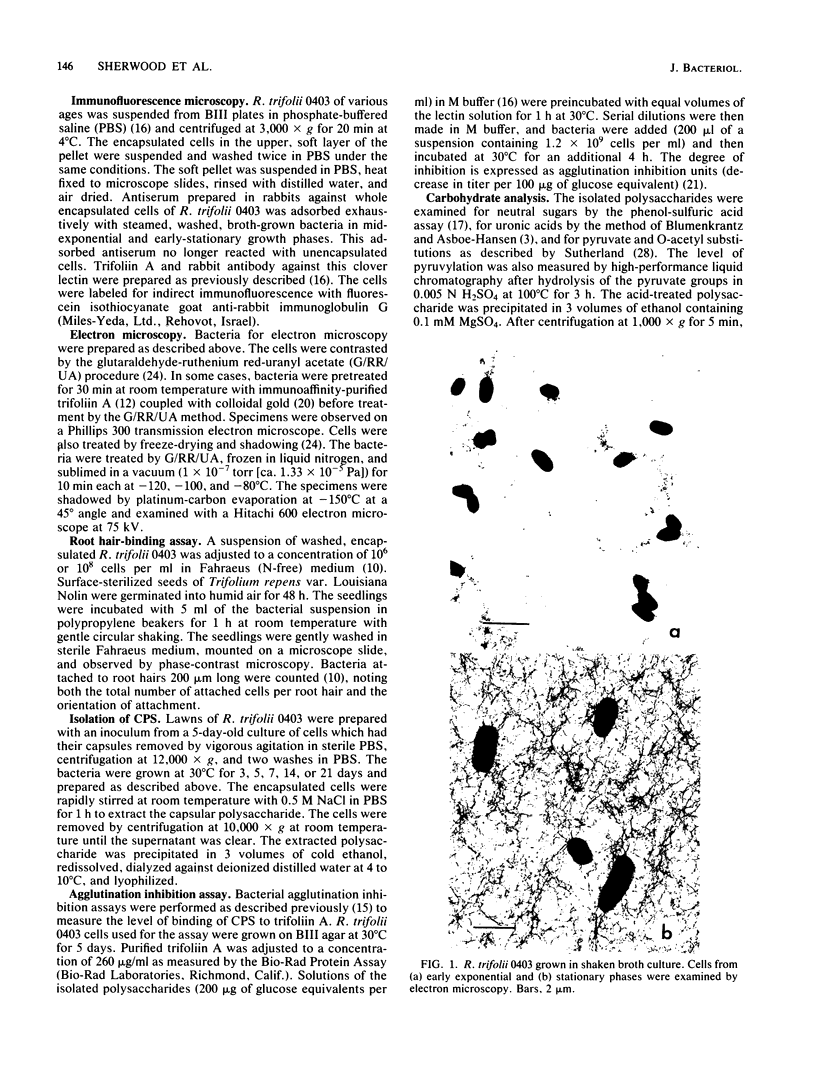
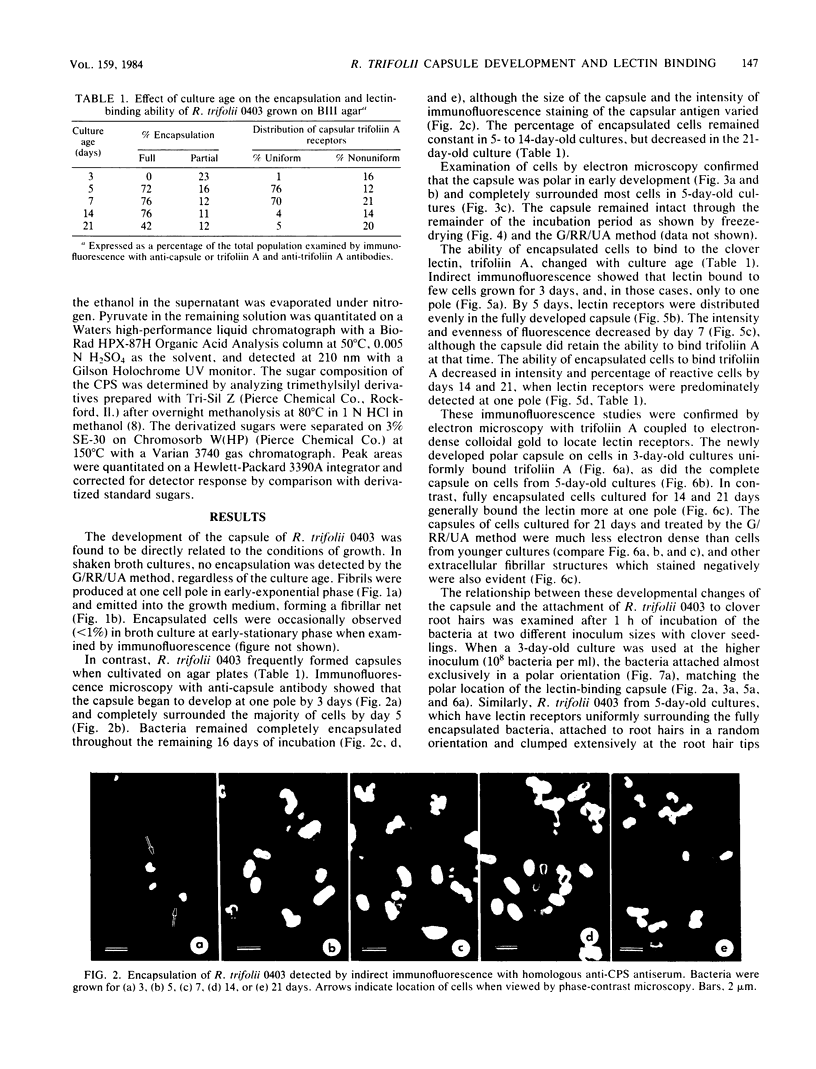
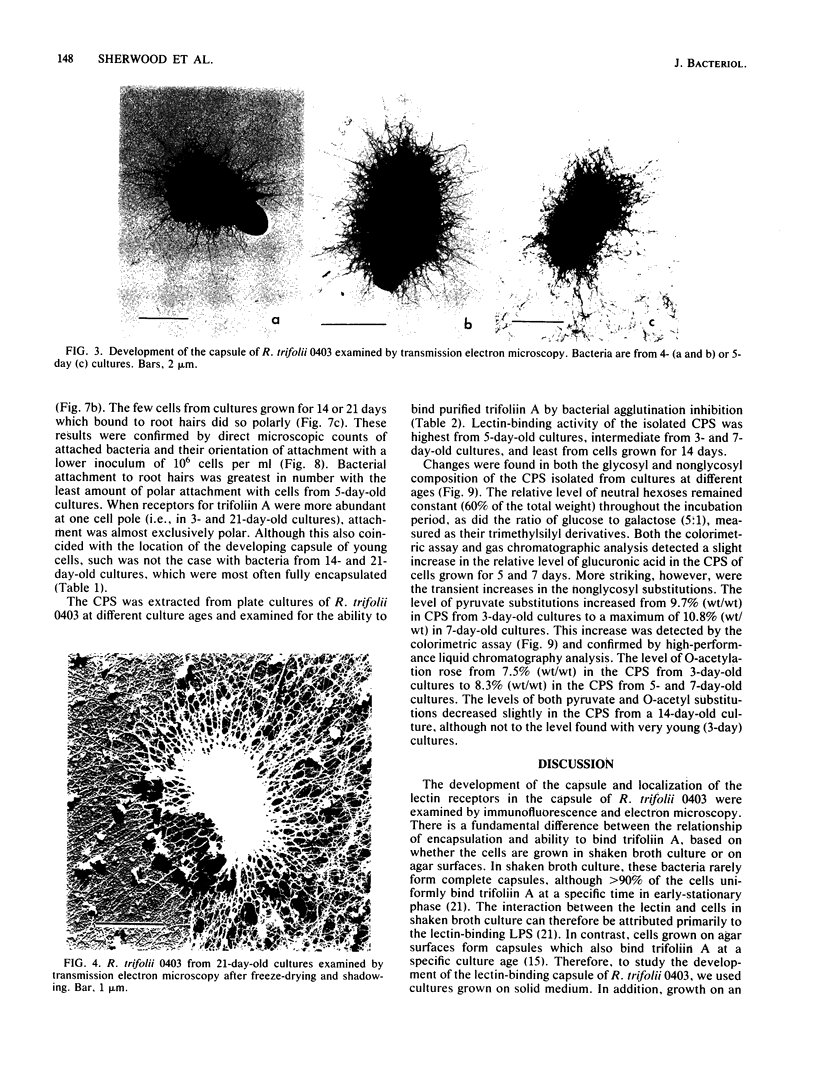
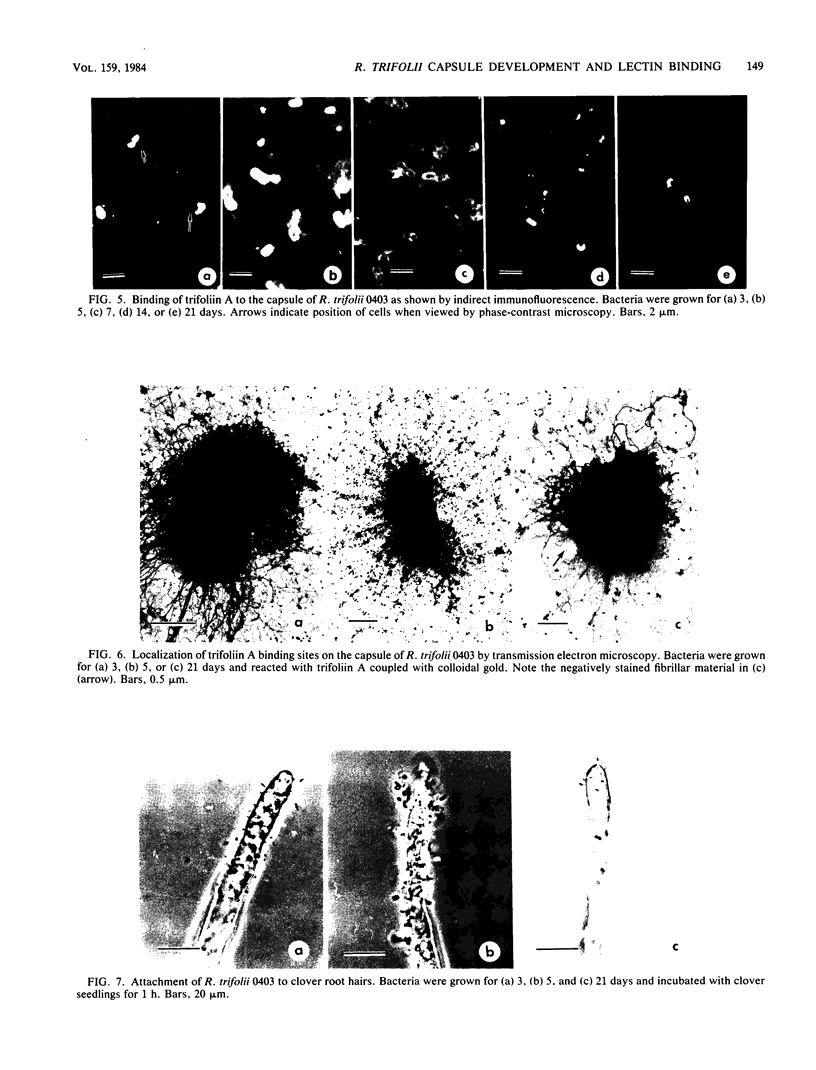
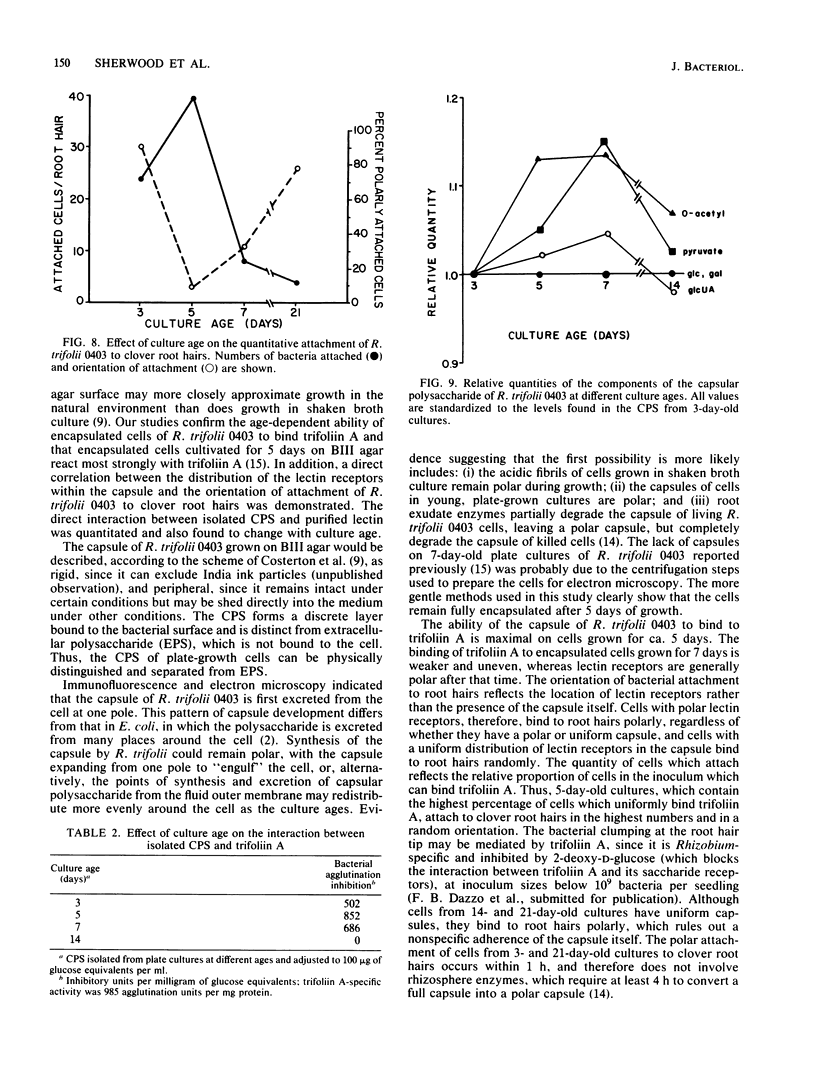
The age-dependent lectin-binding ability of Rhizobium trifolii 0403 capsular polysaccharide (CPS) was examined by following the development of the capsule and its ability to interact with the white clover lectin trifoliin A. Bacteria grown on agar plates for 3, 5, 7, 14, and 21 days were examined by electron microscopy and immunofluorescence microscopy with antibodies prepared against either R. trifolii 0403 CPS or trifoliin A after pretreatment with the lectin. The capsule began to develop at one pole by day 3 and completely surrounded the cells in cultures incubated for 5 days or longer. The capsular polysaccharide on cells cultured for 3 and 5 days was completely reactive with trifoliin A, became noticeably less reactive by day 7, and was only reactive with the lectin at one pole of a few cells after that time. The quantity and location of lectin receptors on bacteria of different ages directly correlated with their attachment in short-term clover root hair-binding studies. Cells from 3- or 21-day-old cultures attached almost exclusively in a polar fashion, whereas cells grown for 5 days attached to root hairs randomly and in the highest numbers. CPS isolated from a 5-day-old culture had higher lectin-binding ability than CPS from 3- and 7-day-old cultures, whereas the CPS from a 14-day-old culture had the lowest. Chemical analyses of the isolated CPS showed changes in the levels of uronic acids (as glucuronic acid), pyruvate, and O-acetyl substitutions with culture age, but the neutral sugar composition remained relatively constant. These results provide evidence that the age-dependent distribution of lectin receptors dictates the level and orientation of attachments of R. trifolii 0403 to clover root hairs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Cadmus M. C., Burton K. A., Slodki M. E. Growth-related substituent changes in exopolysaccharides of fast-growing rhizobia. Appl Environ Microbiol. 1982 Jul;44(1):242–245. doi: 10.1128/aem.44.1.242-245.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Tartagni V. R. Delayed multiplication of newly capsulated transformants of Haemophilus influenzae detected by immunofluorescence. J Gen Microbiol. 1969 Jun;56(3):387–401. doi: 10.1099/00221287-56-3-387. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hrabak E. M. Presence of trifoliin A, a Rhizobium-binding lectin, in clover root exudate. J Supramol Struct Cell Biochem. 1981;16(2):133–138. doi: 10.1002/jsscb.1981.380160204. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Gardiol A. E. Alteration of the Trifoliin A-Binding Capsule of Rhizobium trifolii 0403 by Enzymes Released from Clover Roots. Appl Environ Microbiol. 1982 Aug;44(2):478–490. doi: 10.1128/aem.44.2.478-490.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Dudman W. F. Capsultation in Rhizobium species. J Bacteriol. 1968 Mar;95(3):1200–1201. doi: 10.1128/jb.95.3.1200-1201.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Leps W. T., Brill W. J. Agglutinin from Alfalfa Necessary for Binding and Nodulation by Rhizobium meliloti. Science. 1981 Sep 25;213(4515):1513–1515. doi: 10.1126/science.213.4515.1513. [DOI] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem J. 1969 Dec;115(5):935–945. doi: 10.1042/bj1150935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]