Abstract
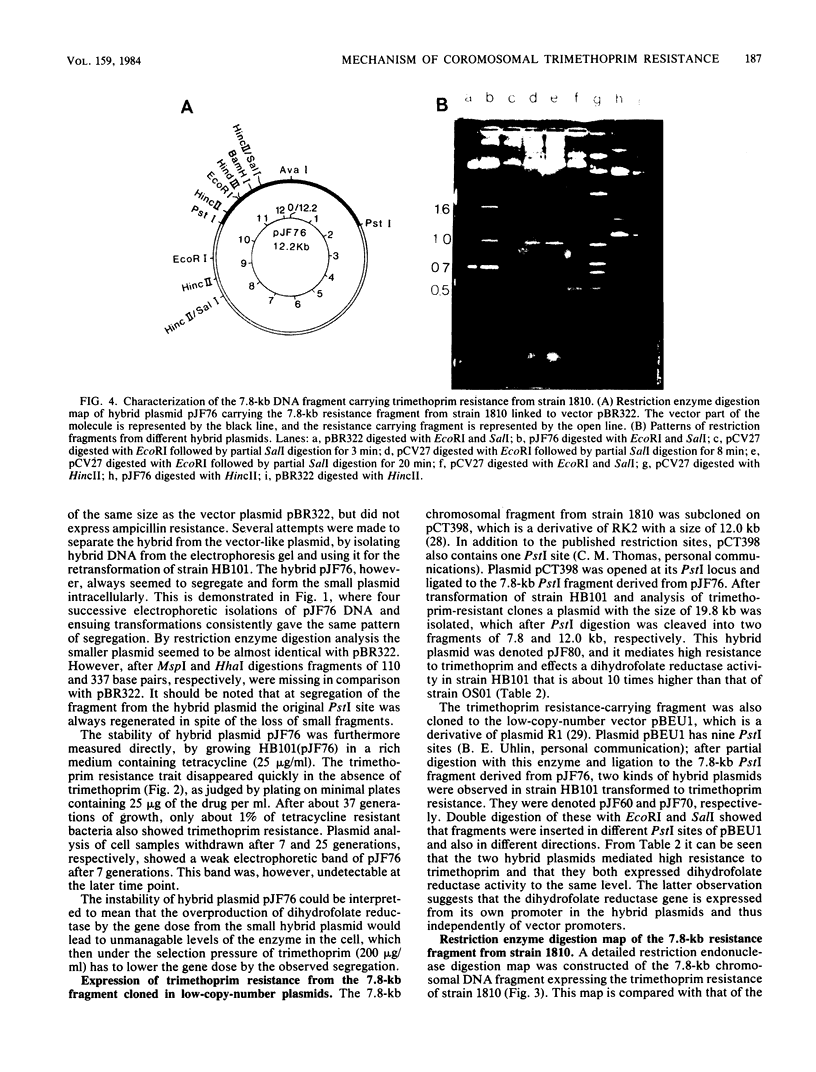
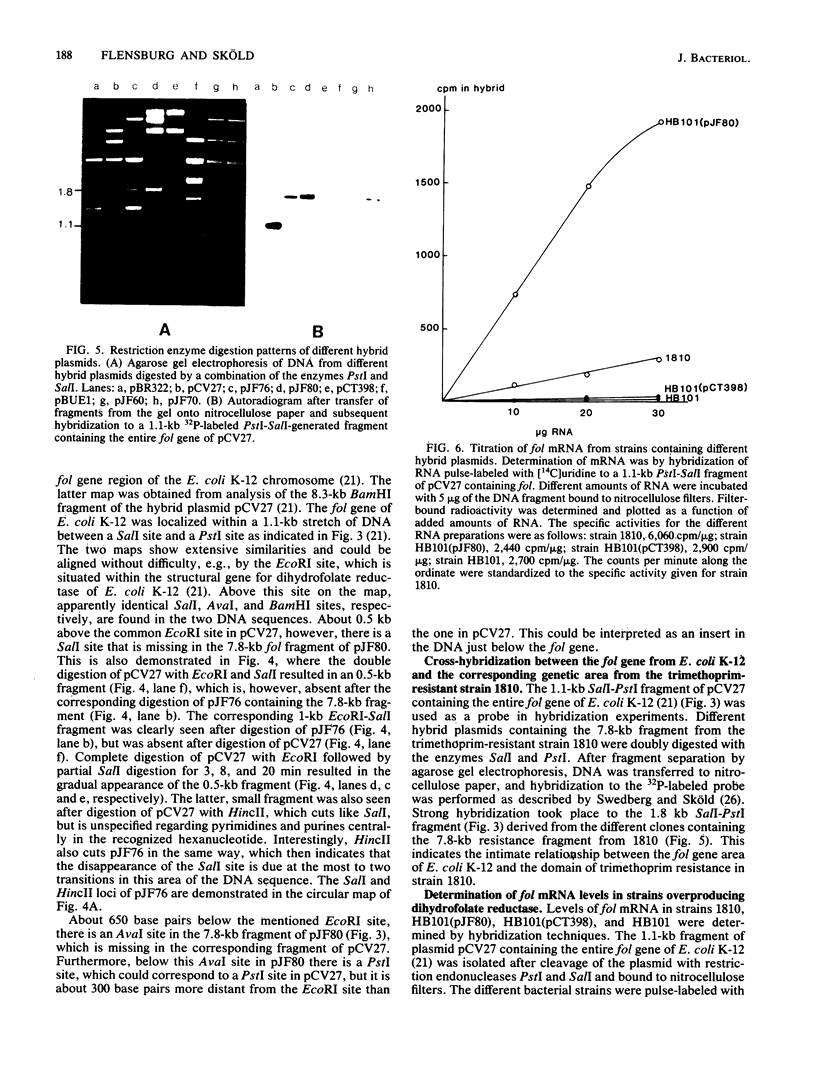
High resistance to trimethoprim mediated by the several hundredfold overproduction of the drug target enzyme, dihyrofolate reductase, in a clinically isolated Escherichia coli strain, 1810, was cloned onto several vector plasmids and seemed to be comprised of a single dihydrofolate reductase gene, which by DNA-DNA hybridization and restriction enzyme digestion mapping was very similar to the corresponding gene of E. coli K-12. Determination of mRNA formation in the originally isolated resistant strain and strains with cloned trimethoprim resistance determinant demonstrated an about 15-fold increase in production of dihydrofolate reductase mRNA compared with that in E. coli K-12. This was explained by the occurrence of a promoter up mutation in the resistant isolate accompanied by changes in the restriction enzyme digestion pattern found by comparison with the corresponding pattern from E. coli K-12.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Edlund T., Grundström T., Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal beta-lactamase gene of Escherichia coli K-12. Mol Gen Genet. 1979 Jun 7;173(2):115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Evolution of antibiotic resistance gene function. Microbiol Rev. 1981 Jun;45(2):355–378. doi: 10.1128/mr.45.2.355-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Burman L. G. Resistance of Escherichia coli to penicillins: fine-structure mapping and dominance of chromosomal beta-lactamase mutations. J Bacteriol. 1977 Oct;132(1):1–7. doi: 10.1128/jb.132.1.1-7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Alt F. W., Kellems R. E., Kaufman R. J., Bertino J. R. Amplification of dihydrofolate reductase genes in methotrexate-resistant cultured mouse cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):649–657. doi: 10.1101/sqb.1978.042.01.067. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Brenner S. Regulatory mutants of dihydrofolate reductase in Escherichia coli K12. Mol Gen Genet. 1976 Aug 10;147(1):91–97. doi: 10.1007/BF00337941. [DOI] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of dihydrofolate reductase genes from trimethoprim-resistant mutants of Escherichia coli. Evidence that dihydrofolate reductase interacts with another essential gene product. Mol Gen Genet. 1982;187(1):72–78. doi: 10.1007/BF00384386. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Regulation of dihydrofolate reductase synthesis in Escherichia coli. Mol Gen Genet. 1979 Aug;175(1):31–38. doi: 10.1007/BF00267852. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Rood J. I., Bird P. I., Sneddon M. K., Calvo J. M., Morrison J. F. Amplification and modification of dihydrofolate reductase in Escherichia coli. Nucleotide sequence of fol genes from mutationally altered plasmids. J Biol Chem. 1982 Aug 10;257(15):9043–9048. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedberg G., Sköld O. Plasmid-borne sulfonamide resistance determinants studied by restriction enzyme analysis. J Bacteriol. 1983 Mar;153(3):1228–1237. doi: 10.1128/jb.153.3.1228-1237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennhammar-Ekman B., Sköld O. Trimethoprim resistance plasmids of different origin encode different drug-resistant dihydrofolate reductases. Plasmid. 1979 Jul;2(3):334–346. doi: 10.1016/0147-619x(79)90017-9. [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Hussain A. A., Smith C. A. Maintenance of broad host range plasmid RK2 replicons in Pseudomonas aeruginosa. Nature. 1982 Aug 12;298(5875):674–676. doi: 10.1038/298674a0. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Clark A. J. Overproduction of the Escherichia coli recA protein without stimulation of its proteolytic activity. J Bacteriol. 1981 Oct;148(1):386–390. doi: 10.1128/jb.148.1.386-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]