Abstract
Previously, we have shown that melanosomes of Xenopus laevis melanophores are transported along both microtubules and actin filaments in a coordinated manner, and that myosin V is bound to purified melanosomes (Rogers, S., and V.I. Gelfand. 1998. Curr. Biol. 8:161–164). In the present study, we have demonstrated that myosin V is the actin-based motor responsible for melanosome transport. To examine whether myosin V was regulated in a cell cycle-dependent manner, purified melanosomes were treated with interphase- or metaphase-arrested Xenopus egg extracts and assayed for in vitro motility along Nitella actin filaments. Motility of organelles treated with mitotic extract was found to decrease dramatically, as compared with untreated or interphase extract-treated melanosomes. This mitotic inhibition of motility correlated with the dissociation of myosin V from melanosomes, but the activity of soluble motor remained unaffected. Furthermore, we find that myosin V heavy chain is highly phosphorylated in metaphase extracts versus interphase extracts. We conclude that organelle transport by myosin V is controlled by a cell cycle-regulated association of this motor to organelles, and that this binding is likely regulated by phosphorylation of myosin V during mitosis.
Keywords: myosin, molecular motors, melanophores, melanosomes, regulation
Cytological studies of many different types of organelles in animal cells have revealed that their proper spatial and temporal distribution relies upon transport along elements of the cytoskeleton. The Golgi apparatus, for example, is restricted to its perinuclear position in many differentiated cell types by transport to the minus ends of microtubules by cytoplasmic dynein (Corthesy-Theulaz et al. 1992; Burkhardt et al. 1997). In contrast, the steady-state distribution of the ER in the cell's periphery is actively mediated by transport to the distal, plus ends of microtubules by kinesin (Feiguin et al. 1994). Other types of organelles are more dynamic and must be transported to specific intracellular destinations to perform their specific tasks. Secretory vesicles travel from the Golgi apparatus, their point of origin, to the plasma membrane for exocytosis (Lafont and Simons 1996). Endosomes are transported centripetally from the cell's periphery to its interior for subsequent processing (Bomsel et al. 1990). In neurons, synaptic vesicles move from the cell body to sites of synaptic connections, which may be up to meters away (Amaratunga et al. 1993), while mitochondria are believed to be transported up gradients of ADP to intracellular sites of high ATP consumption (Nangaku et al. 1994). Perhaps the most dramatic example of organelle transport occurs during cell division when, during prophase, chromosomes are transported along microtubules to congress at the metaphase plate, and later, during anaphase, segregate to their respective, opposite poles (Waters and Salmon 1997).
Many years of study have led to the dogma that the majority of intracellular transport occurs along microtubules, driven by the activities of the dynein and kinesin superfamilies of motor proteins. However, in recent years, this assumption has been challenged by the demonstration that some organelles can move along filamentous actin (Adams and Pollard 1986; Kuznetzov et al. 1992; Bearer et al. 1993; Mermall et al. 1994; Morris and Hollenbeck 1995; Krendel et al. 1998). Compelling evidence that a single type of organelle can be transported along both microtubules and actin filaments has resulted from the study of pigment granule transport in melanophores, a model system for the study of intracellular transport. The role of these cells, present in the dermal layers of fish and amphibians, is to cyclically aggregate pigmented organelles, termed melanosomes, to the center of the cell or disperse them throughout the cytoplasm to effect color changes in the animal's skin (Haimo and Thaler 1994). In Xenopus laevis melanophores, pigment transport is regulated by hormone-induced modulation of intracellular cAMP levels: melanocyte-stimulating hormone (MSH)1 triggers dispersion by upregulation of cAMP production, while melatonin induces pigment aggregation by downregulating cAMP levels (Daniolos et al. 1990). This hormone-induced organelle transport is regulated by antagonistic cycles of kinase and phosphatase activities (Reilein et al. 1998). Until recently, it was believed that melanosomes were exclusively carried along the cells' radially organized microtubule cytoskeleton with a kinesin-related protein, kinesin-II, transporting pigment to the microtubule plus ends during dispersion and dynein moving them to the minus ends during aggregation (Nilson and Wallin 1997; Tuma et al. 1998). It is now clear, however, that another, actin-based component also contributes to pigment transport in melanophores. Upon disruption of the microtubule cytoskeleton, melanosomes exhibit short, shuttling movements that halt in the presence of actin-depolymerizing drugs (Rodionov et al. 1998). Furthermore, we have demonstrated that purified melanosomes can move along actin filaments in vitro and that the actin-based motor, myosin V, is associated with these organelles (Rogers and Gelfand 1998). Similar findings of coordinated actin- and microtubule-based transport were also reported for melanosomes in cultured mouse melanocytes (Wu et al. 1998a).
The mitotic cell is confronted with the important task of ensuring that both daughter cells receive their appropriate allotment of each organelle type (Warren 1993; Warren and Wickner 1996; Shima et al. 1998). Since the interphase distributions of many organelles rely upon the activities of motor proteins, it stands to reason that their segregation during mitosis must be accompanied by modulation of the activities of associated motors. At present, the Allan and Vale laboratories have performed the only studies directly examining this topic. Using Xenopus frog egg extracts arrested in metaphase, these groups demonstrated that both plus and minus end directed microtubule-based transport of membranous organelles was inactivated (Allan and Vale 1991). Furthermore, mitotic inhibition of dynein-mediated organelle transport is achieved by dissociation of the motor from its cargo, and this dissociation correlated with phosphorylation of the motor by a mitotic kinase activity (Niclas et al. 1996).
Previous studies of mitotic melanophores in vivo documented that these cells do not respond to stimuli which normally induce pigment aggregation and dispersion in interphase, suggesting that melanosomal motors may, indeed, be differentially regulated throughout the cell cycle (Starobudov and Golichenkov 1988). Melanophores provide a very useful system to study motor protein regulation. The melanosomes present in these cells may be purified rapidly and in large quantities, and have been shown to exhibit both microtubule- and actin-based motility in vitro. Treatment of isolated melanosomes with Xenopus egg extracts arrested either in metaphase or interphase allows the study of cell cycle-dependent regulation of the microtubule- and actin-based motors present on these organelles. In this study, we have demonstrated that myosin V is the motor responsible for actin-based transport of melanosomes in Xenopus melanophores through the use of a dominant-negative myosin V construct and by immunofluorescent localization of the motor to melanosomes. We then used our system to study the regulation of myosin V during mitosis. Treatment of melanosomes with metaphase, but not interphase, extracts resulted in a dramatic decrease in vitro motility. This decreased motility was due to dissociation of myosin V from pigment granules and not due to inhibition of its motor activity. The myosin V heavy chain exhibited a substantial increase in phosphate incorporation in mitotic extracts, compared with interphase extracts, implicating phosphorylation of myosin V as the regulatory mechanism. To our knowledge, this is the first study documenting a molecular mechanism for the cell cycle-mediated regulation of actin-based organelle transport.
Materials and Methods
Melanophore Cell Culture and Transfection
Immortalized Xenopus melanophores were cultured as described previously (Rogers et al. 1997). Immunofluorescent localization of myosin V was performed using a clonal nonpigmented cell line, clone 47, or gray cells, derived from the original melanophore cell line (Daniolos et al. 1990). Melanophores containing a lower melanin content were selected by freezing the original cell line in 95% FCS and 5% DMSO, according to standard protocols. Approximately 5% of the cells survived thawing and reculturing, many of them possessing large vesicles containing small (∼0.2 μm) particles of melanin. This cycle of freezing and thawing was repeated once again and pigment-deficient cells were cloned twice on 10-cm tissue culture plates using the cloning ring technique. A morphologically stable clone was selected and expanded. Over 50% of the cells in this population contained numerous unmelanized vesicles ∼1-μm diam. These vesicles responded to hormone treatment by aggregating and dispersing as normal melanosomes and were, therefore, considered pigment-free melanosomes. Further melanin production was inhibited by treating the cells for 2 wk with 1 mM phenylthiourea. Cultures isolated in this manner were grown in standard growth medium, supplemented with 1 mM phenylthiourea.
To preserve cellular morphology for microscopy, cells were transfected using the FuGENE 6 transfection reagent (Boehringer Mannheim Corp.) following the vendor's protocols.
Construction of myc-tagged Headless Myosin V
To prepare the plasmid pcDNA3-Myc-MST, which contains the COOH-terminal 601 amino acids of the mouse myosin Va gene fused to the COOH terminus of the myc epitope tag (Evan et al. 1985), the following PCR primers were constructed: 5′-AAA AAG CTT AAA CCA TGG AGC AAA AGC TCA TTT CTG AAG AGG ACC TGG GGA TCC AAG CTG-3′ and 5′-AAA CTC GAG TCA GAC CCG TGC GAT GAA-3′ (GIBCO BRL), and used to amplify the myosin short tail DNA from the construct pCMV2-FLAG-MC-ST (Wu et al. 1998a). The product of this PCR was digested with XhoI and HindIII, and cloned into the vector pcDNA3 (Invitrogen Corp.).
Immunofluorescence Microscopy
Melanophores were plated on acid-washed polylysine-coated glass coverslips and cultured for 24 h. Cells were then briefly rinsed in 0.7× PBS and fixed for 20 min in a solution of freshly prepared 3% paraformaldehyde in 0.7× PBS. A solution of 0.1% Triton X-100 was used to permeabilize cells for 15 min. For immunofluorescent staining, cells were blocked using 3% BSA in the same solution for 10 min. Myc epitope-tagged proteins were stained using the 1-9E10.2 mAb (Evan et al. 1985) diluted 1:1,000 into the BSA/Triton buffer for 60 min. Myosin V distribution was visualized with the DIL2 polyclonal antibody (see below) at a dilution of 1:8,000. The cells were then washed with PBS, stained using FITC-conjugated goat anti–mouse secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) diluted 1:100 for 60 min, washed with 0.7× PBS, and mounted in 80% glycerol in 0.1 M sodium borate, pH 8, supplemented with N-propyl gallate. For fluorescent actin staining, rhodamine-conjugated phalloidin (Molecular Probes, Inc.) was diluted to 0.33 nM and included with the secondary antibodies. Images were obtained using a Zeiss Axioskop equipped with a CH250 cooled CCD camera (Princeton Instruments). The gray-scale histograms of the images were stretched to utilize their full dynamic ranges using Adobe Photoshop.
Preparation of Xenopus Egg Extracts and Endogenous Egg Organelles
Interphase and metaphase-arrested Xenopus egg extracts were prepared as described (Murray 1991; Allan 1993), with the following modifications. CSF-arrested frog eggs were activated by treatment with 10 μM A23187 for 5 min and allowed to progress to interphase in the presence of 100 μg/ml cycloheximide before extract preparation. Metaphase-arrested extracts were prepared by treating interphase extracts with 0.13 mg/ml bacterially expressed Δ90, a nondegradable cyclin B construct, for 45 min at room temperature (Glotzer et al. 1991). All extracts were supplemented with 5 μM latrunculin A (BIOLMOL) to depolymerize actin (Spector et al. 1989). Histone H1 kinase assays were used to monitor activity of cdc2/p34 kinase activity during every experiment (Allan 1993).
High-speed supernatants were prepared by centrifuging egg extracts at 150,000 g in a TLA 100.3 rotor (Beckman Instruments, Inc.) for 30 min at 4°C. Endogenous egg organelles were isolated by floatation as described in Lane and Allan 1999.
Treatment of Melanosomes with Xenopus Egg Extracts
Melanosomes were isolated from cultured melanophores, essentially as described in Rogers et al. 1998. In brief, melanophores were grown in 10-cm tissue culture plates to confluency. Cells were rinsed with IMB50 (50 mM imidazole, pH 7.4, 1 mM magnesium acetate, 1 mM EGTA, 150 mM sucrose, 0.5 mM EDTA, 1 mM dithiothreitol, 150 μg/ml casein, 1 mM ATP) then scraped into 2 ml of the same buffer supplemented with protease inhibitors (10 μg/ml each chymostatin, leupeptin, and pepstatin, and 1 mM PMSF). All further manipulations were performed on ice. Cells were lysed by 5 to 10 passes through a 26-gauge needle attached to a 1 ml hypodermic syringe and diluted to 10 ml. The lysate was centrifuged at 600 g in an HB-6 rotor (Sorvall) for 5 min to remove nuclei and unbroken cells. The supernatant was recovered and centrifuged again for 5 min at 2,000 g in an HB-6 rotor to pellet melanosomes. Melanosome pellets were then gently resuspended either in Xenopus egg extracts (melanosomes from two plates per 150 μl extract) or IMB50 supplemented with 1 mM ATP as a control. After a 30 min incubation at room temperature, melanosome/extract mixtures were diluted to 1 mL with IMB50 and layered atop a 5-ml cushion of 80% Percoll in IMB50 and purified by centrifugation at 2,000 g for 10 min in an HB-6 rotor. Melanosomes to be used in motility assays were resuspended in 100 μl IMB50 supplemented with 1 mM ATP. Those destined for SDS-PAGE and immunoblotting were dissolved in 20 μl of sample buffer.
Egg extracts were immunodepleted using either affinity-purified DIL2 (see below) or preimmune serum obtained from the same rabbits, following a published protocol described in Desai et al. 1998.
SDS-PAGE and Immunoblotting
Electrophoretic separation of proteins was routinely performed using discontinuous 7.5% SDS-PAGE gels (Laemmli 1970). In experiments comparing protein composition between different treatments of melanosomes, purified pigment granules were resuspended in sample buffer, boiled for 5 min, and equivalent volumes of organelles were diluted into 1% SDS. The absorbance of melanin at 550 nm was determined for each sample and this measurement was used to normalize the load in each lane to an equivalent number of organelles. Immunoblotting was performed using the protocol of Towbin et al. (Towbin et al. 1979) and bound antibody was detected by chemiluminescence using SuperSignal (Pierce Chemical Co.).
Myosin V was detected using a polyclonal antibody, DIL2, raised against bacterially expressed neck domain of the dilute isoform of mouse myosin V, and affinity-purified as described (Wu et al. 1997). Kinesin-II was detected using an mAb cross-reactive with the motor's 85-kD subunit, K2.4 (Cole et al. 1993). The intermediate chain of cytoplasmic dynein was probed with an mAb, m74-1 (Steffen et al. 1997).
Nitella In Vitro Motility Assays
The Nitella-based motility assay was performed essentially as described (Sheetz et al. 1986), with the following modifications. Before dissection, each Nitella internodal cell was treated with 2 mM N-ethyl maleimide for 5 min to poison the plant's endogenous cytoplasmic streaming and ensure that all motility observed was due to the activity of melanosome-associated myosin. Nitella cells were then dissected into buffer containing 2 mM dithiothreotol, 2 mM ATP, and 10 μM phalloidin to stabilize filamentous actin. Melanosomes or protein A beads were then pipetted onto dissected Nitella filets and observed using time-lapse video-enhanced bright-field microscopy with a 40× long-working distance objective mounted on a Diaphot 300 inverted microscope (Nikon, Inc.). Images were captured using a Newvicon camera and an Argus-10 video processor (Hamamatsu Phototonics) and recorded onto s-VHS tapes with a time-lapse video recorder (Panasonic).
To examine the activity of myosin V in egg extracts, protein A–agarose beads (Sigma Chemical Co.) were incubated with affinity-purified DIL2 polyclonal antibody at a protein concentration of 25 μg/ml, or with preimmune serum for 1 h at 4°C. The beads were then washed in IMB50 by resuspension and centrifugation five times to remove unbound antibody. The preimmune-bound bead pellets (∼50 μl vol) were then resuspended in 200 μl of interphase or metaphase egg extracts clarified of membranes by centrifugation at 150,000 g in a TLA 100.3 rotor (Beckman Instruments, Inc.) to preclear for 1 h at 4°C. The beads were pelleted, and each aliquot of extract was then incubated either with DIL2- or preimmune-conjugated beads for 1 h at 4°C. After washing five times in IMB50 supplemented with 2 mM ATP, these beads were used in the Nitella assay, as described above.
32P-labeling and Immunoprecipitation of Myosin V from Egg Extracts
Xenopus egg extracts arrested in interphase or metaphase were labeled with 200 μCi 32Pi in 20 μl reactions and incubated for 30 min at room temperature. Reactions were stopped by the addition of 1 ml ice-cold IP buffer (10 mM Tris, 80 mM sodium β-glycerophosphate, 10 mM sodium pyrophosphate, pH 7.5) supplemented with 1% Triton X-100, and a protease inhibitor cocktail (10 μg/ml each leupeptin, pepstatin, and chymostatin, and 1 mM PMSF) and incubated on ice for 10 min. The samples were clarified by centrifugation at 16,000 g for 15 min and precleared for 90 min by incubation with normal rabbit serum, prebound to 25 μl of a 50% protein A–agarose bead suspension. After centrifugation, 25 μl of 0.5 mg/ml affinity-purified DIL2 antibody was added and incubated for 4 h at 4°C. The samples were then incubated with 30 μl of protein A beads for 30 min, and the beads were collected by centrifugation at 10,000 g for 10 min. The following regimen of washes was then performed: four times with ice-cold IP buffer, one time with IP buffer supplemented with 500 mM sodium chloride, and once with 50 mM Tris, pH 8. Pellets were resuspended in 20 μl sample buffer and analyzed by SDS-PAGE and autoradiography.
Results
Myosin V Is Responsible for Melanosome Transport Along Actin Filaments
Using an in vitro assay, we previously demonstrated the presence of an actin-based motor on the surface of melanosomes purified from Xenopus melanophores (Rogers and Gelfand 1998). Tentative identification of this motor as myosin V was based on the observation that this motor is enriched in purified melanosome fractions relative to whole cell extracts, while members of several other myosin classes were absent. To determine conclusively whether myosin V was actually involved in melanosome transport in this system, we sought to disrupt its function in melanophores and to observe the effects on pigment granule transport. Since latrunculin A-induced disruption of the actin cytoskeleton in Xenopus melanophores results in microtubule-dependent aggregation of the pigment to the cell center (Rogers and Gelfand 1998), we hypothesized that inhibition of the melanosome-associated myosin would produce the same effect. Melanophores were, therefore, transfected with a construct encoding an epitope-tagged fragment of mouse myosin Va (Wu et al. 1998a). This fragment, lacking the NH2-terminal motor domain, consists of the COOH-terminal 601 amino acids of myosin V, and includes part of the central stalk domain believed to be important in homodimerization of myosin V heavy chains by coiled-coil interaction, and the globular tail domain, thought to be the cargo-binding site. This construct, termed myosin V short tail (MST), has been shown to act as a dominant-negative inhibitor of myosin V in mouse melanocytes; its expression mimicking the naturally occurring genetic-null phenotype in this cell type (Wu et al. 1998a).
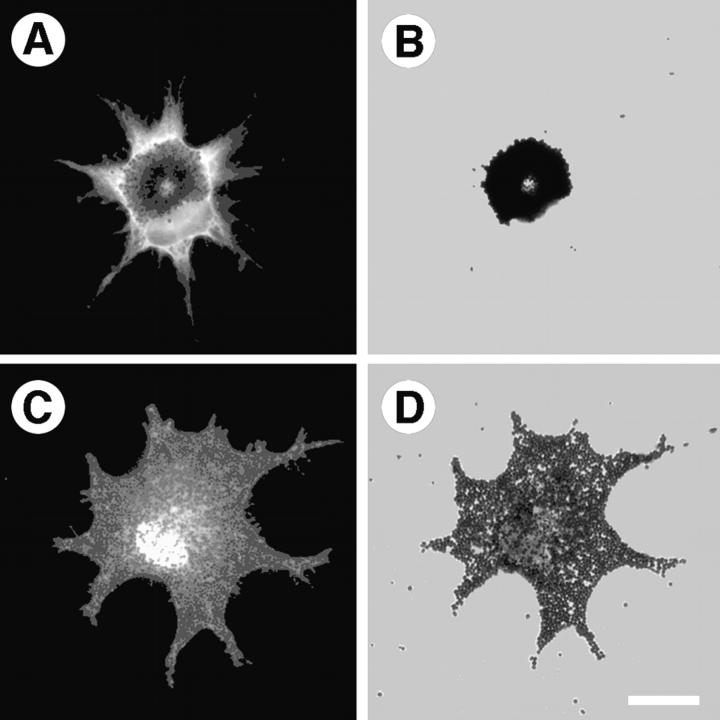
Melanophores were transfected with the MST construct; 48 h later cultures were fixed and transfected cells identified by immunofluorescent staining for the myc epitope-tag present at the NH2 terminus of the mutant construct. Xenopus melanophores usually grow in culture with their melanosomes dispersed throughout their cytoplasm unless induced to aggregate to a tight, central mass by treatment with melatonin. Without exception, every cell expressing MST aggregated its pigment to the cell center (n = 250; Fig. 1A and Fig. B). Immunolocalization of expressed myc-tagged MST revealed that the protein was present throughout the entire volume of the cell. Melanosome aggregation was not due to a disruption of the actin cytoskeleton, as staining with fluorescent phalloidin revealed a filamentous actin distribution in these cells, which was similar to nontransfected cells (data not shown). Control cells transfected with green fluorescent protein (GFP) underwent cycles of pigment aggregation and dispersion, indicating that pigment aggregation was an effect specifically caused by MST (Fig. 1C and Fig. D).
Figure 1.
Expression of the dominant-negative MST construct induces uninduced pigment aggregation in melanophores. A, Transfected cell stained with an mAb against the myc epitope-tag to verify expression of MST. Expressed MST is present throughout the cytoplasm, but staining in the center of the cell is obscured by the pigment mass. B, This is a bright-field image of the cell in A, demonstrating that its pigment is aggregated to the center of the cell. C, Control cells transfected with GFP grow in culture with dispersed pigment (D). Bar, 20 μm.
To verify the involvement of myosin V in melanosome transport, we sought to determine whether or not the motor was present on melanosomes in situ by immunofluorescent staining. The chemical properties of melanin make this approach problematic, however, for two reasons. First, melanin is a polymer composed of modified tyrosine residues and is rife with charged carboxyl and nonpolar aromatic side chains (Sarna 1992). Previous studies have documented the high affinity of melanin for various compounds, and it has been our experience that melanin avidly binds many proteins present in cell extracts (Rogers et al. 1998). Second, as melanin has been evolved to act as a light-absorbing pigment, it interferes with fluorescence microscopy, especially in aggregated cells where the pigment mass often occludes staining. To circumvent these problems, we developed a melanin-free clonal melanophore subline. The original Xenopus melanophore cell line was subjected to two freeze–thaw cycles to select for melanophores possessing a low melanin-content. After two rounds of cloning, a morphologically stable cell line was isolated. Any residual melanin was eliminated by culturing the cells in 1 mM phenylthiourea, a potent tyrosinase inhibitor, for two weeks. This cell line, designated clone 47 or gray cells, possesses numerous vesicles which are ∼1 μm in diameter, and respond to treatment with MSH and melatonin by dispersing to the cell periphery or aggregating to the cell center (Fig. 2A and Fig. B). Therefore, we believe that these vesicles are melanosomes devoid of pigment.
Figure 2.
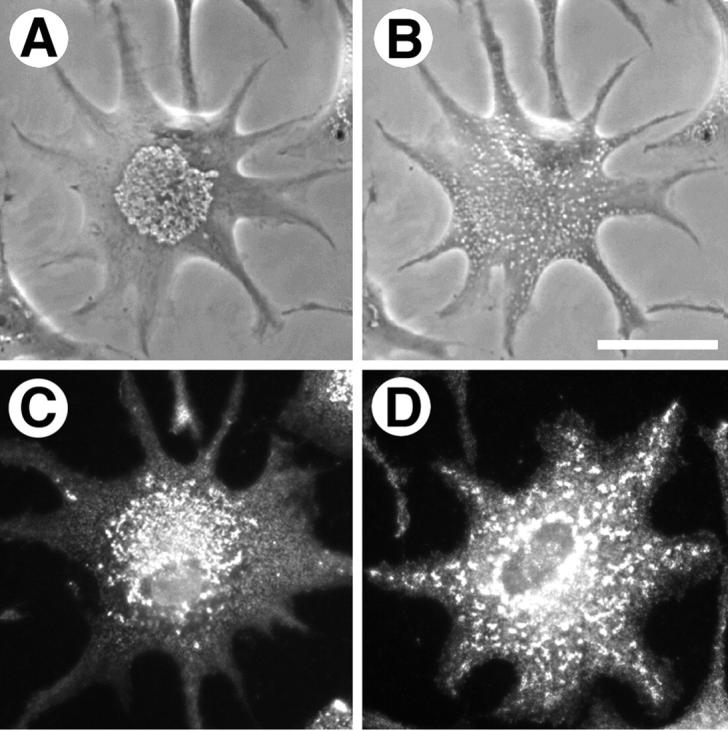
Phase-contrast microscopy of an amelanotic melanophore possessing numerous vesicles that aggregate to the center of the cell upon treatment with melatonin (A) and disperse following treatment with MSH (B). In these cells, myosin V immunolocalizes to unmelanized melanosomes and comigrates with these organelles during aggregation (C) and dispersion (D). Bar, 20 μm.
Gray cells were fixed and immunofluorescently stained with an antibody specific for myosin V. The antibody was highly enriched on punctate vesicular structures that aggregated to the cell center upon treatment with melatonin (Fig. 2 C) and dispersed throughout the cells after exposure to MSH (Fig. 2 D). We conclude that myosin V is present on melanosomes in vivo in Xenopus melanophores and remains bound to these organelles, at least to some degree, during aggregation and dispersion.
Cell Cycle-dependent Regulation of Myosin V
Previous studies examining Xenopus melanophores in situ have shown that these cells fail to transport their pigment in response to MSH or melatonin during mitosis (Starobudov and Golichenkov 1988). Work from our lab has established that melanosome transport occurs along both the microtubule and actin cytoskeletons in a coordinated manner (Rogers and Gelfand 1998). We considered the possibility that the melanosome-associated motors are differentially regulated throughout the cell cycle. Since nothing is known about whether actin-mediated organelle transport is regulated during cell division, we chose to focus upon this question by treating melanosomes with Xenopus egg extracts arrested in metaphase or interphase and examining their motility in vitro.
We initially prepared Xenopus egg extracts arrested in interphase or metaphase essentially according to the protocols of Murray 1991, as modified by Allan 1993. Frog eggs were activated using calcium ionophore in the presence of cytochalasin D to prevent actin polymerization. Cycloheximide was included to arrest them in interphase, and extracts were obtained by centrifugal crushing. Extracts at this stage were found to remain stably in interphase. Metaphase arrested-extracts were prepared by further supplementing these extracts with the nondegradable cyclin derivative, Δ90 (Glotzer et al. 1991). Mitotic extracts were found to stably maintain high MPF kinase activity (data not shown). Both interphase and metaphase extracts prepared this way completely inhibited actin-based motility of melanosomes in vitro using the Nitella assay, as compared with untreated control organelles. Biochemical studies of purified myosin V demonstrated that this motor binds to actin with high affinity, even in the presence of ATP (Nascimento et al. 1996). Therefore, we speculated that cytochalasin-capped fragments of actin filaments bound to myosin V present on the melanosomes, effectively blocking the motor from interacting with exogenous Nitella actin filaments. To circumvent this possibility, latrunculin A, a drug which binds to monomeric actin and, unlike cytochalasin, induces complete depolymerization of filamentous actin, was included during extract preparation (Spector et al. 1989).
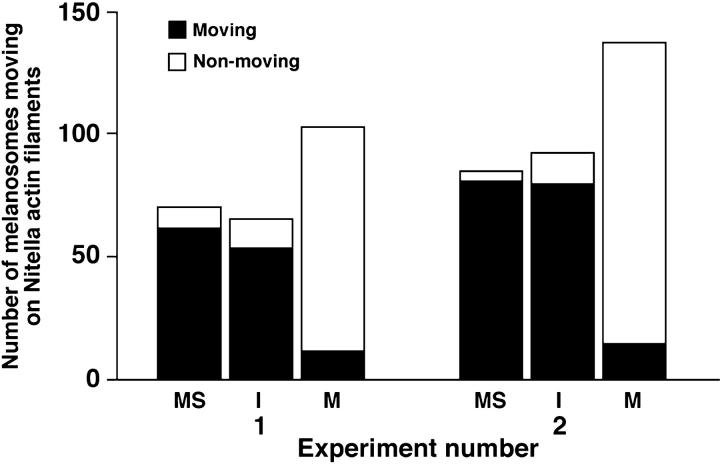
To establish a basal level of melanosome motility, organelles were purified from melanophores and scored for their ability to move in vitro using the Nitella assay. In agreement with our previous results (Rogers and Gelfand 1998), we found that ∼90% of the total number of pigment granules exhibited unidirectional motility (Fig. 3). When isolated, melanosomes were preincubated in interphase frog egg extracts supplemented with latrunculin and purified by density gradient centrifugation. The fraction of organelles transported along actin filaments was ∼85%, virtually indistinguishable from the untreated control. However, treatment with metaphase-arrested extracts dramatically inhibited actin-based motility; only ∼10% of the melanosomes exhibited motility in vitro. Incubation of the melanosomes with metaphase extracts, therefore, decreased motility nearly eightfold, compared with interphase extracts or untreated organelles.
Figure 3.
Treatment of melanosomes with metaphase-, but not interphase-arrested frog egg extracts inhibits actin-based motility along Nitella actin filaments in vitro. The histogram shows the numbers of motile (black bar) and immotile (white bar) melanosomes treated with interphase extracts (I), metaphase extracts (M), or untreated control organelles (MS). Treatment with mitotic extracts inhibited motility ∼10-fold, compared with the other two treatments. The results of two independent experiments are shown.
Myosin V Dissociates from Melanosomes in Metaphase-arrested Frog Egg Extracts
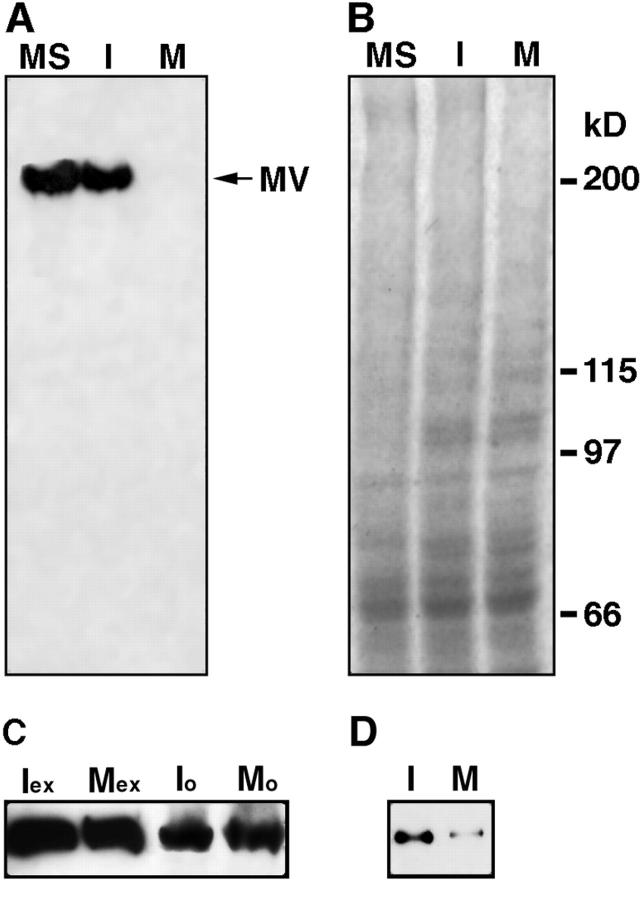
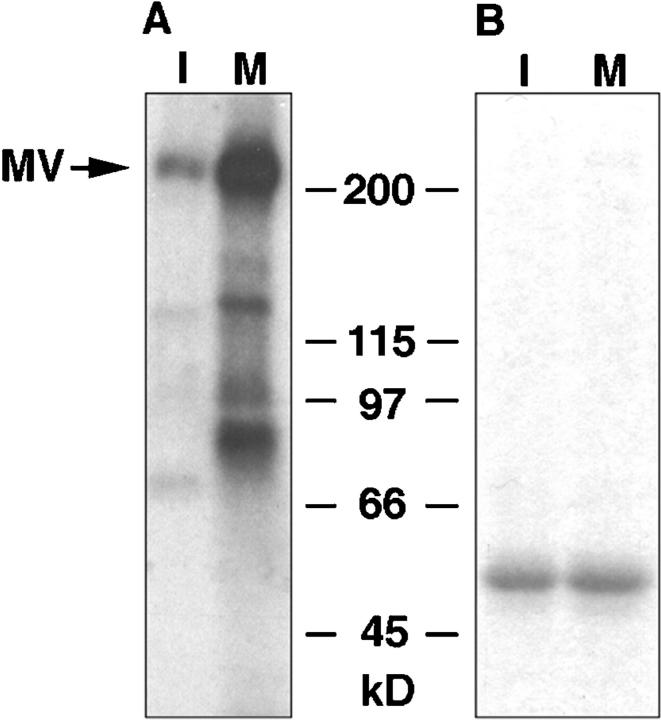
We hypothesized that the inhibition of motility observed following metaphase extract treatment could be due to one of two possible mechanisms: myosin V may dissociate from melanosomes or the motor could be rendered inactive during mitosis. To test this first possibility, melanosomes were isolated, treated with either interphase- or metaphase-arrested extracts, and examined by electrophoresis and immunoblotting for myosin V (Fig. 4 A). The number of melanosomes in each sample was normalized by optical density to ensure that an equal number of organelles were analyzed for each treatment. In addition, Coomassie blue staining of gels run in parallel also verified the amount of protein loaded per treatment (Fig. 4 B). Immunoblotting revealed that untreated melanosomes contained the same amount of myosin V as interphase extract-treated organelles. However, in melanosome fractions treated with metaphase extracts, myosin V was undetectable. To verify that the source of the myosin V we were detecting was from melanosomes, and not from endogenous egg organelles that might fuse or aggregate with pigment granules, extracts were clarified by high-speed centrifugation before melanosome treatment. High-speed mitotic supernatants were as effective in release of myosin V from melanosomes as extracts prepared by low-speed centrifugation (Fig. 4 D).
Figure 4.
A, Immunoblot for myosin V of melanosome fractions treated with mitotic extracts (M), interphase extracts (I), or untreated organelles (MS). Myosin V is absent from metaphase treated organelles. The samples for each lane were normalized to load equivalent numbers of melanosomes by measuring the absorbance of melanin at 550 nm. Myosin V is designated by MV. B, Coomassie blue stained gel of the samples shown for A, demonstrating that the protein loaded in each lane is approximately equal. C, Immunoblot for myosin V in interphase extracts (Iex), metaphase extracts (Mex), interphase organelles (Io), and metaphase organelles (Mo) from frog extracts. Equal amounts of protein were loaded for each sample. D, Immunoblot for myosin V on melanosomes treated with interphase (I) and metaphase (M) high-speed supernatants prepared from egg extracts.
Immunoblots for myosin V revealed that the motor was present in both mitotic- and interphase-arrested egg extracts in approximately equal amounts (Fig. 4 C). This indicated that the protein was not subjected to proteolytic degradation in a cell cycle-dependent manner.
Endogenous egg organelles from both types of extracts were purified by flotation through a sucrose gradient to exclude soluble proteins and analyzed for the presence of myosin V (Fig. 4 C). Interestingly, the motor was found to remain associated to these organelles in both interphase and metaphase extracts. This result suggests that the dissociation of myosin V may be an organelle-specific phenomenon. Furthermore, it conclusively rules out the possibility that melanosome fractions became contaminated with endogenous organelles during treatment with the egg extracts. We noted that the mobility of myosin V bound to organelles did not exhibit a shift in molecular weight in either population of organelles or as compared with soluble myosin V from either type of extract.
If melanosome-bound myosin V was able to exchange with the soluble pool of egg-derived motor, then our observations might also reflect a cell cycle-dependent association of egg myosin V with melanosomes. To test whether this was the case, we immunodepleted myosin V from both interphase- and metaphase-arrested extracts before melanosome addition. We reasoned that if there were an exchange between the organelles and the extracts, then we would detect a net release from interphase-treated melanosomes in myosin V-depleted extracts. Immunoblots of organelles treated with mitotic extract depleted of myosin V lacked the motor, whereas immunodepleted interphase extracts retained myosin V (data not shown). However, when we compared the relative amount of myosin V bound to melanosomes after treatment with motor-depleted extracts with organelles treated with extracts immunodepleted using preimmune serum, we noted a quantitative difference. In myosin V-depleted extracts, melanosomes retained less motor, indicating that egg-derived myosin V was able to exchange with the melanosome-bound protein in interphase-arrested extracts (data not shown). These results indicate that melanosomal myosin V is able to exchange with myosin V derived from interphase extracts, but neither the egg or melanosomal myosin can bind to organelles in metaphase-arrested cytosol.
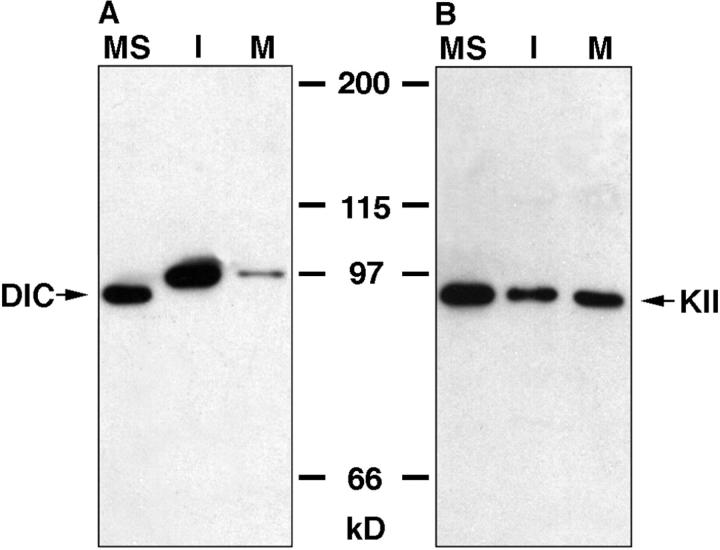
Previous work from our lab has demonstrated that the bidirectional transport of pigment granules along microtubules in Xenopus melanophores is due to the activities of the plus-end directed motor, kinesin-II, and the minus end directed motor, cytoplasmic dynein (Tuma et al. 1998; Karcher, R., and V. Gelfand, unpublished data). Niclas et al. 1996 demonstrated that dynein-driven motility of Golgi membranes and ER membranes exhibits cell cycle-dependent regulation in Xenopus mitotic egg extracts, and this inhibition is due to dissociation of the motor. Our system allowed us to test if this is a general phenomenon for dynein-driven motility. Consistent with the results of Niclas et al. 1996, quantitative immunoblotting for dynein using an antibody raised against the intermediate chain demonstrated that this motor is released from melanosomes in metaphase extracts (Fig. 5 A). Interestingly, dynein intermediate chain in extract-treated melanosome fractions exhibits a mobility shift of ∼10 kD, compared with its apparent molecular weight of 83 kD on untreated melanosomes. This mobility shift has been observed by other groups who have attributed it to represent posttranslational modification (Huang et al. 1999) or the recruitment of an egg-specific isoform of the motor (Lane and Allan 1999). In an effort to distinguish between these two possibilities, we treated egg extracts with alkaline phosphatase to determine whether this mobility shift was due to phosphorylation. The migration of dynein intermediate chain remained unaffected by this treatment (data not shown), indicating that melanosomes likely recruited an egg-specific isoform. Kinesin-II was found to remain associated with metaphase-extract–treated pigment granules by immunoblotting with an antibody that recognizes the 85-kD subunit of the motor (Fig. 5 B).
Figure 5.
Immunoblots of microtubule motors present on metaphase-treated (M), interphase-treated (I), or untreated (MS) melanosomes. A, Detection of cytoplasmic dynein by immunoblotting for dynein intermediate chain (DIC) with the m74-1 mAb. Dynein is dissociated from melanosomes in mitotic frog egg extract. B, Kinesin-II (KII) was detected in similarly treated melanosomes with an antibody against the motor's 85-kD motor subunit, the monoclonal K2.4. The amounts of this motor on melanosomes are unaffected by treatment with extracts. The load for each lane was equalized for the number of melanosomes by absorbance.
Our experiments with the dominant-negative myosin V construct demonstrated that the activity of this motor is necessary for proper dispersion of melanosomes in melanophores. It is, therefore, possible that modulation of myosin V's activity is a key regulatory event during the cycles of aggregation and dispersion within these cells. Since elevation of intracellular cAMP and the subsequent activation of protein kinase A (PKA) plays a key role in triggering pigment dispersion, we considered the possibility that the activity of this kinase may play a role in the association of myosin V with melanosomes throughout progression of the cell cycle. The activity of PKA in cycling Xenopus egg extracts has been extensively studied, and has been found to play an important role during the transition of mitosis to interphase (Greico et al. 1996). The basal level of cAMP production and PKA activity was found to decrease during the transition from interphase to mitosis, and to exhibit a peak in activity just before the transition to interphase (Greico et al. 1994). If PKA activity were essential for myosin V attachment to melanosomes, then the diminished activity of the kinase in metaphase-arrested extracts might account for the motor's dissociation. To test this hypothesis, we incubated purified melanosomes in metaphase egg extracts in the presence of 50 μM cAMP and 1 mM 1-isobutyl 3-methyl xanthine to inhibit phosphodiesterases. Comparison of immunoblots of treated versus untreated metaphase extracts showed no difference in the amount of myosin V present on melanosomes; the motor dissociated from the organelles in both cases (data not shown). Addition of the PKA catalytic subunit to metaphase extracts, likewise, did not prevent this dissociation (data not shown). Mitotic release of myosin V from melanosomes was, therefore, not due to the inactivity of PKA.
The Motor Activity of Myosin V Is Not Differentially Regulated Throughout the Cell Cycle
In addition to dissociation from its cargo, another potential regulatory mechanism for myosin V could be inhibition of its motor activity. To test this possibility, affinity-purified myosin V antibody was bound to protein A-conjugated agarose beads. The beads were then used to isolate myosin V from interphase- and metaphase-arrested egg extracts that had been clarified of membranes by ultra-centrifugation. Beads with attached myosin V were analyzed in the Nitella motility assay.
Myosin V immunoisolated on beads from interphase (Fig. 6 A) and mitotic (Fig. 6 B) extracts exhibited vigorous motility in the Nitella assay. Virtually every bead from both samples exhibited motility, with average velocities of 84.2 ± 19 nm/s for interphase beads and 52.8 ± 17 nm/s for metaphase beads. These velocities are somewhat faster than those observed for untreated melanosomes in vitro (41 ± 20 nm/s). Melanosomes treated with interphase extracts exhibited average velocities of 32.8 ± 7.8 nm/s, while metaphase treated melanosomes traveled at 27.8 ± 10.5 nm/s. Beads conjugated with preimmune serum from the same rabbit in which the DIL2 antibodies were generated exhibited no motility, indicating that the movements we observed were due to immunoadsorbed myosin V. We conclude that the motor activity of myosin V is not inhibited in mitotic extracts.
Figure 6.
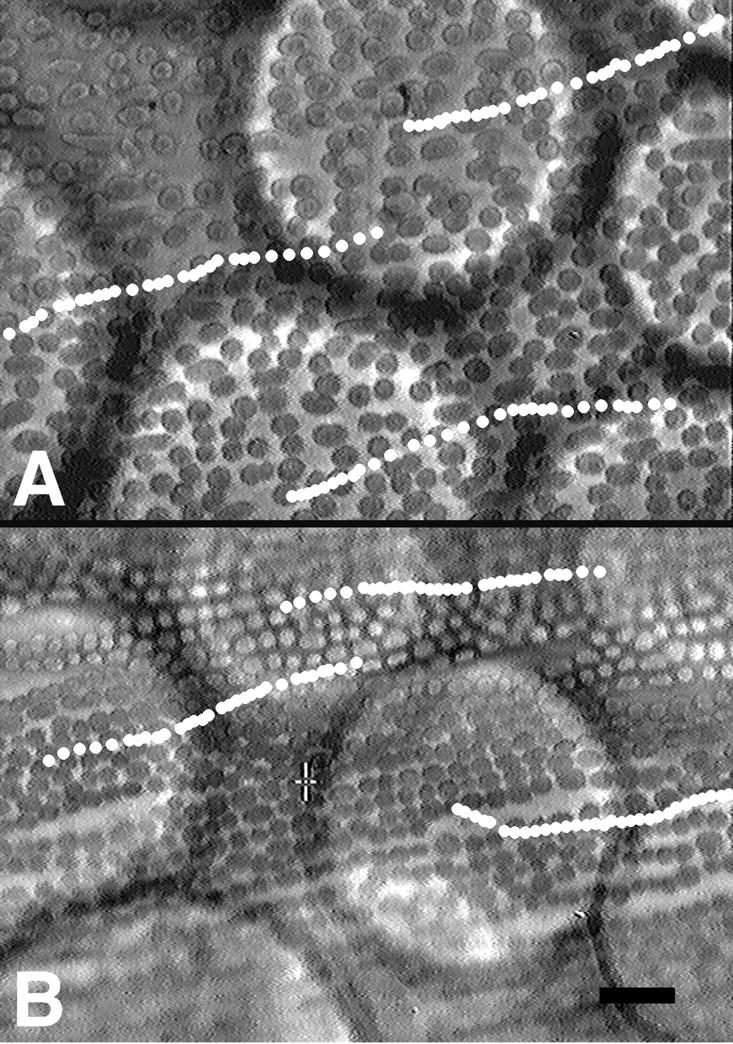
Immunoadsorbed myosin V from interphase (A) or metaphase (B) extracts is able to transport Sepharose beads in the Nitella motility assay. The beads appear as large, refractile spheres, whereas the smaller oval objects are the underlying Nitella chloroplasts. The centers of three beads in each panel were tracked over 30 min; each white dot overlaid on the video frames represents the position of the beads in 1 min increments. Bar, 20 μm.
Phosphorylation of Myosin V in Metaphase Extracts
Since many cellular processes, including dynein-mediated transport, are modulated in a cell cycle-dependent manner by phosphorylation, we tested the possibility that myosin V might be regulated similarly. Interphase- and metaphase-arrested egg extracts were treated with [32P]orthophosphate to produce an endogenous pool of labeled ATP to act as a substrate for kinases present in the extracts. Myosin V was then immunoprecipitated and equal amounts of protein were analyzed by electrophoresis and autoradiography. In both interphase and metaphase extracts, a 200-kD myosin V heavy chain was immunoprecipitated and found to be phosphorylated (Fig. 7). Quantitation of the amount of radioactive phosphate incorporated into the protein in each treatment revealed that in mitotic cytosol it was labeled approximately fivefold greater than in interphase extract. Myosin V is, therefore, more highly phosphorylated in metaphase, compared with interphase. Interestingly, several additional phosphoproteins coimmunoprecipitated with myosin V. In interphase extracts, proteins with molecular weights of 70 and 20 kD exhibited labeling, while in metaphase extracts, species of 85, 100, 120, and 140 kD remained associated (Fig. 7). The molecular weights of these proteins do not correlate with any known subunits of this motor, and the significance of these possible associations is unknown.
Figure 7.
Phosphorylation of myosin V in cell cycle-arrested frog egg extracts. A, Extracts were 32P-labeled, and myosin V was immunoprecipitated from interphase- and metaphase-arrested extracts using affinity-purified DIL2 antibody (I and M, respectively), separated by SDS-PAGE, and analyzed by autoradiography. Myosin V (MV) is more highly phosphorylated in metaphase extracts. B, Coomassie blue stained gel as in A, to demonstrate approximately equal protein load for both treatments.
The metaphase-arrested extracts that we used were prepared by treatment of interphase extracts with Δ90, a sea urchin cyclin B construct modified to delete its ubiquitinization sequence (Glotzer et al. 1991). Δ90 associates with and activates cdc2/p34 kinase, driving the extracts into metaphase, but since the cyclin's degradation sequence has been removed, the kinase activity of the cyclin B/p34 complex remains constitutively active and the extracts cannot progress further. We speculated that myosin V might be phosphorylated directly by cdc2/p34 mitotic kinase. To test this hypothesis, we purified melanosomes and treated them in vitro with commercially available recombinant cyclin B/cdc2 kinase. After the melanosomes were repurified by density gradient centrifugation, immunoblotting for myosin V showed that the motor continued to remain associated with the organelles in amounts similar to untreated controls (data not shown). Furthermore, when phosphorylated in the presence of 32P, no significant amount of isotope was observed to incorporate into any melanosome proteins in the molecular weight range expected of myosin V (data not shown). Finally, phosphorylation by cyclin B/cdc2 kinase did not affect the amount of melanosome motility in the Nitella assay, as compared with untreated control organelles. Histone H1 was employed as a control substrate in these experiments to show that cdc2 was active under our experimental conditions in the presence of melanosomes. We conclude that metaphase-induced phosphorylation of myosin V is not directly due to cdc2/p34, but rather to the activity or activities of a different kinase or phosphatase, or both, which exhibit differential activities in metaphase extracts.
Discussion
It has become evident over the past several years that cells partition membrane-bound organelles to their daughters by precisely regulated, yet unique, mechanisms. Intracellular membranes undergo a specific choreography within the spindles of living PtK2 cells, first collecting along the microtubules to gather at the poles through prometaphase, followed by an abrupt exclusion from the spindle at metaphase (Waterman-Storer et al. 1993). Using a GFP-labeled resident Golgi apparatus protein, Shima et al. documented the fragmentation of the Golgi apparatus in living HeLa cells during mitosis and observed that the resultant vesicles segregate to each daughter cell via a static association with each pole of the spindle in a microtubule-dependent process (Shima et al. 1997, Shima et al. 1998). GFP-labeled peroxisomes, which are transported vectorally along microtubules during interphase, lose their association with the cytoskeleton during cell division and appear to be segregated randomly (Weimer et al. 1997). In frog melanophores, melanosomes are excluded from the mitotic spindle and fail to respond to the hormonal stimuli that induce them to aggregate or disperse during interphase (Starobudov and Golichenkov 1988). One common feature between these examples is the apparent downregulation of microtubule-based transport during metaphase. This hypothesis is borne out by the work of the Allan and Vale laboratories, which demonstrated that dynein-mediated microtubule minus end-directed transport, along with plus end transport, of the ER and Golgi membranes are inhibited in metaphase-arrested Xenopus egg extracts (Allan and Vale 1991; Niclas et al. 1996).
In the present study, we examined whether myosin V was subjected to cell cycle-dependent regulation. Our previous work demonstrated that melanosomes purified from Xenopus melanophores exhibit actin-based motility in vitro (Rogers and Gelfand 1998). Furthermore, the unconventional myosin, myosin V, was found to be enriched in melanosome fractions, compared with whole melanophore extracts, suggesting that this motor is responsible for movement along actin filaments. In this study we have confirmed this hypothesis both in vivo and in vitro by using a dominant-negative approach to block myosin V function and by immunolocalizing myosin V to melanosomes in unpigmented melanophores. To test cell cycle-governed regulation of myosin V bound to melanosomes, we prepared Xenopus egg extracts arrested either in interphase or metaphase. Melanosomes incubated in interphase extracts and untreated organelles exhibited vigorous motility in the Nitella assay, but mitotic-treated melanosomes showed an eightfold decrease in their in vitro movement. Furthermore, this mitotic inhibition was caused by dissociation of myosin V from melanosomes without an accompanying inhibition of its motor activity. We used a polyclonal antibody raised against a 27-kD fragment of myosin V for detection of this motor and do not believe that our inability to detect it on blots of metaphase-treated melanosomes is due to masking of the epitope by posttranslational modification. Membranous organelles purified from egg extracts did not exhibit cell cycle-induced dissociation, however, suggesting that mitotic release of myosin V may be specific for certain organelles. Interestingly, we did observe a slight difference in the velocities of soluble myosin V between interphase and mitotic extracts, which may indicate a second level of regulation of this motor. Dissociation of myosin V was accompanied by increased phosphorylation of its heavy chain in mitotic extracts, relative to interphase extracts. Therefore, we propose a mechanism whereby myosin V-driven organelle transport is inactivated during mitosis by phosphorylation-induced dissociation from melanosomes.
Although myosin V is one of the best characterized unconventional myosins, its regulation is poorly understood. To date, only one other study has addressed this issue directly. Prekeris and Terrian 1997 demonstrated a calcium-induced release of myosin V from synaptic vesicles in vitro, as well as in isolated synaptosomes (Prekeris and Terrian 1997). They showed that this dissociation occurred in the absence of ATP and was, therefore, not due to phosphorylation of the motor. We believe that the metaphase-induced release of myosin V from melanosomes occurs through a different regulatory mechanism for two reasons. First, in our study, melanosomes and Xenopus egg extracts were prepared in the presence of the calcium chelator, EGTA. Second, treatment of purified melanosomes with exogenous calcium failed to affect the amount of myosin V bound to the organelles (data not shown). Tissue-specific isoforms of myosin V are produced as the result of differential RNA splicing; brain and epidermal myosin V each possess different protein domains as a result (Seperack et al. 1995). It is possible that this difference in calcium sensitivity may be specific to the neuronal isoform. Alternatively, it may be that organelle receptor proteins for the motor respond to different signals; those on synaptic vesicles, synaptophysin and synaptotagmin II, release myosin V upon exposure to calcium, whereas the unidentified receptor on melanosomes does not (Prekeris and Terrian 1997).
It is interesting to compare the regulatory mechanisms that govern cytoplasmic dynein with those of myosin V. Although both transport membrane-bound organelles along different cytoskeletal filaments during interphase, they dissociate from their cargo during mitosis. Cytoplasmic dynein has been implicated in other processes during cell division, such as spindle formation, chromosome transport, and spindle orientation (Waters and Salmon 1997; Palazzo et al. 1999). It is possible that dynein dissociates from its membrane-bound organelle cargo so that it may be recruited to perform these other tasks during mitosis. Alternatively, it may also be that the motor is subjected to identical regulatory mechanisms during interphase and mitosis, but specificity of the cargo transported by dynein changes during the cell cycle, and this differential targeting is modulated during mitosis. A recent study of the distribution of myosin V during mitosis has shown that this motor is present in the spindle and midbody of dividing cells, suggesting that it too may play a role during mitosis (Espreafico et al. 1998; Wu et al. 1998b). However, the fact that dilute myosin V null mice do not exhibit gross mitotic defects suggests that if it plays a role in cell division, it is either nonessential or it is compensated by other factors (Searle 1952).
The best understood example of unconventional myosin regulation has resulted from the study of ameboid myosin I. Myosins IA, IB, and IC from Acanthamoeba all exhibit increased actin-based motility and ATPase activity upon phosphorylation of a conserved serine or threonine present in an actin-binding loop in the motors' heads (Brzeska and Korn 1996; Carragher et al. 1998). Bement and Mooseker, after subjecting all known myosin sequences to a comprehensive sequence comparison, noted that in all other known myosins, except for myosin VI, this residue is replaced with either glutamate or aspartate (Bement and Mooseker 1995). This observation led them to postulate the TEDS rule, in which they hypothesized that the requirement for phosphorylation on this residue in the majority of myosins may have been evolutionarily relieved by the substitution of an acidic amino acid residue (Bement and Mooseker 1995). If this is true, then it is logical to assume that differential regulation of other unconventional myosins, such as myosin V, could be achieved by differential attachment the motors to their cargoes. This argument is borne out in the case of myosin V by the results of our study, as well as those of Prekeris and Terrian 1997. Other mechanisms should not be excluded, however. Myosin V is a phosphoprotein in nervous tissue and is a substrate for calcium–calmodulin activated kinase II in vitro (Larson et al. 1988, Larson et al. 1990). We have shown here that myosin V is phosphorylated in interphase egg extracts, albeit at lower levels than in metaphase-arrested extracts, without affecting the amount of motor bound to melanosomes. This observation suggests the possibility that the motor possesses multiple phosphorylation sites, which may be modified differentially throughout the cell cycle. The significance of this phosphorylated state is unknown, however, and the physiological relevance of these observations has yet to be established. In addition to the dissociation of myosin V from synaptic vesicles, in vitro studies of the motor have demonstrated that its motility is inhibited by calcium and this inhibition is likely due to loss of myosin V-associated calmodulin light chains (Cheney et al. 1993). Paradoxically, calcium treatment also increases the motor's actin-stimulated ATPase activity (Nascimento et al. 1996). Intracellular modulation of calcium levels also may be a potential mechanism of myosin V function.
Acknowledgments
We are grateful to Dr. John Hammer III and Dr. Xufeng Wu for generously providing us with the myosin V short tail reagents. We thank Dr. Ann Marie Craig for the generous use of her microscope. Thanks also to Alex Szidon, Dr. Andrew Murray, and Dr. Frank McNally for advice and reagents for egg extract preparation. We are also grateful to Dr. Li Ma and Dr. Marc Kirschner for originally supplying extracts during the initial stages of this study.
This work was supported by grants from the National Science Foundation (MCB-951338) and the National Institutes of Health (GM-52111) to V.I. Gelfand, and the Howard Hughes Medical Institute (75195-544704) to A.A. Minin. W. Steffen was supported by a grant from the Fond zur Förderung Wissenschaftlicher Forschung (P12868-GEN). The work of A.A. Minin in the laboratory of V.I. Gelfand was supported by the National Science Foundation.
Footnotes
1.used in this paper: GFP, green fluorescent protein; MSH, melanocyte-stimulating hormone; MST, myosin V short tail construct; PKA, protein kinase A
References
- Adams R., Pollard T. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin I. Nature. 1986;322:754–756. doi: 10.1038/322754a0. [DOI] [PubMed] [Google Scholar]
- Allan V. Assay of membrane motility in interphase and metaphase Xenopus extracts. Methods Cell Biol. 1993;39:203–226. [PubMed] [Google Scholar]
- Allan V.J., Vale R.D. Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J. Cell Biol. 1991;113:347–359. doi: 10.1083/jcb.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga A., Morin P., Kosik K., Fine R. Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J. Biol. Chem. 1993;268:17427–17430. [PubMed] [Google Scholar]
- Bearer E., Degiorgis J., Bodner R., Kao R., Reese T. Evidence for myosin motors on organelles in squid axoplasm. Proc. Natl. Acad. Sci. USA. 1993;90:11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W., Mooseker M. TEDS rulea molecular rationale for differential regulation of myosins by phosphorylation of the heavy chain head. Cell Motil. Cytoskeleton. 1995;31:87–92. doi: 10.1002/cm.970310202. [DOI] [PubMed] [Google Scholar]
- Bomsel M., Parton R., Kuznetsov S., Schroer T., Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Brzeska H., Korn E. Regulation of class I and class II myosins by heavy chain phosphorylation. J. Biol. Chem. 1996;271:16983–16986. doi: 10.1074/jbc.271.29.16983. [DOI] [PubMed] [Google Scholar]
- Burkhardt J.K., Echeverri C.J., Nilsson T., Vallee R.B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher B., Cheng N., Wang Z., Korn E., Reilein A., Belnap D., Hammer J., Steven A. Structural invariance of constitutively active and inactive mutants of Acanthamoeba myosin IC bound to F-actin in the rigor and ADP-bound states. Proc. Natl. Acad. Sci. USA. 1998;95:15206–15211. doi: 10.1073/pnas.95.26.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney R.E., O'Shea M.K., Heuser J.E., Coelho M.V., Wolenski J.S., Espreafico E.M., Forscher P., Larson R.E., Mooseker M.S. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Cole D., Chinn K., Wedaman K., Hall T., Vuong T., Scholey J. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- Corthesy-Theulaz I., Pauloin A., Pfeffer S. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniolos A., Lerner A., Lerner M. Action of light on frog pigment cells in culture. Pigment Cell Res. 1990;3:38–43. doi: 10.1111/j.1600-0749.1990.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Desai A., Murray A., Mitchison T., Walczak C. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell. Biol. 1998;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Espreafico E.M., Coling D.E., Tsakraklides V., Krogh K., Wolenski J.S., Kalinec G., Kachar B. Localization of myosin-V in the centrosome. Proc. Natl. Acad. Sci. USA. 1998;95:8636–8641. doi: 10.1073/pnas.95.15.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G., Lewis G., Ramsay G., Bishop J. Isolation of monoclonal antibodies specific for c-myc proto-oncogene product. Mol. Cell. Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F., Ferreira A., Kosik K., Caceres A. Kinesin-mediated organelle transport revealed by specific cellular manipulations. J. Cell Biol. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A., Kirschner M. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Greico D., Avvedimento E., Gottesman M. A role for cAMP-dependent protein kinase in early embryonic divisions. Proc. Natl. Acad. Sci. USA. 1994;91:9896–9900. doi: 10.1073/pnas.91.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greico D., Porcellini A., Avvedimento E., Gottesman M. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271:1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- Haimo L., Thaler C. Regulation of organelle transportlessons from color change in fish. BioEssays. 1994;16:727–733. [Google Scholar]
- Huang C., Chang C., Huang C., Ferrell J. M-phase phosphorylation of cytoplasmic dynein intermediate chain and p150Glued . J. Biol. Chem. 1999;274:14262–14269. doi: 10.1074/jbc.274.20.14262. [DOI] [PubMed] [Google Scholar]
- Krendel M., Sgourdas G., Bonder E.M. Disassembly of actin filaments leads to increased rate and frequency of mitochondrial movement along microtubules. Cell Motil. Cytoskeleton. 1998;40:368–378. doi: 10.1002/(SICI)1097-0169(1998)40:4<368::AID-CM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kuznetzov S., Langford G., Weiss D. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafont F., Simons K. The role of microtubule-based motors in the exocytic transport of polarized cells. Semin. Cell Dev. Biol. 1996;7:343–356. [Google Scholar]
- Lane J., Allan V. Microtubule-based endoplasmic reticulum motility in Xenopus laevisactivation of membrane-associated kinesin during development. Mol. Biol. Cell. 1999;10:1909–1922. doi: 10.1091/mbc.10.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R., Pitta D., Ferro J. A novel 190 KDa calmodulin-binding protein associated with brain actomyosin. Braz. J. Med. Biol. Res. 1988;21:213–217. [PubMed] [Google Scholar]
- Larson R., Espindola F., Espreafico E. Calmodulin-binding proteins and calcium/calmodulin-regulated enzyme activities associated with brain actomyosin. J. Neurochem. 1990;54:1288–1294. doi: 10.1111/j.1471-4159.1990.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Mermall V., McNally J., Miller K. Transport of cytoplasmic particles catalysed by an unconventional myosin in living Drosophila embryos. Nature. 1994;369:560–562. doi: 10.1038/369560a0. [DOI] [PubMed] [Google Scholar]
- Morris R., Hollenbeck P. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nangaku M., Yoshitake R.S., Okada Y., Noda Y., Takemura R., Yamazaki H., Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nascimento A.A.C., Cheney R.E., Tauhata S.B.F., Larson R.E., Mooseker M.S. Enzymatic characterization and functional domain mapping of brain myosin-V. J. Biol. Chem. 1996;271:17561–17569. doi: 10.1074/jbc.271.29.17561. [DOI] [PubMed] [Google Scholar]
- Niclas J., Allan V., Vale R. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J. Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson H., Wallin M. Evidence for several roles of dynein in pigment transport in melanophores. Cell Motil. Cytoskeleton. 1997;38:397–409. doi: 10.1002/(SICI)1097-0169(1997)38:4<397::AID-CM9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Palazzo R.E., Vaisberg E.A., Weiss D.G., Kuznetsov S.A., Steffen W. Monoclonal antibody to dynein intermediate chain inhibits spindle assembly in cytoplasmic extracts of Spissula solidissima . J. Cell Sci. 1999;112:1291–1302. doi: 10.1242/jcs.112.9.1291. [DOI] [PubMed] [Google Scholar]
- Prekeris R., Terrian D. Brain myosin V is a synaptic vesicle-associated motor proteinevidence for a Ca2+-dependent interaction with the synaptobrevin–synaptophysin complex. J. Cell Biol. 1997;137:1589–1601. doi: 10.1083/jcb.137.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilein A., Tint I., Peunova N., Enikolopov G., Gelfand V. Regulation of organelle movement in melanophores by protein kinase A (PKA), protein kinase C (PKC), and protein phosphatase 2A (PP2A) J. Cell Biol. 1998;142:803–813. doi: 10.1083/jcb.142.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov V., Hope A., Svitkina T., Borisy G. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr. Biol. 1998;8:165–168. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- Rogers S., Gelfand V. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- Rogers S., Tint I., Fanapour P.G., Gelfand V. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc. Natl. Acad. Sci. USA. 1997;94:3720–3725. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Tint I., Gelfand V. In vitro motility assay for melanophore pigment organelles. Methods Enzymol. 1998;298:361–372. doi: 10.1016/s0076-6879(98)98032-6. [DOI] [PubMed] [Google Scholar]
- Sarna T. Properties and function of the ocular melanina photophysical view. J. Photochem. Photobiol. B. 1992;12:215–258. doi: 10.1016/1011-1344(92)85027-r. [DOI] [PubMed] [Google Scholar]
- Searle A. A lethal allele of dilute in the house mouse. Heredity. 1952;6:395–401. [Google Scholar]
- Seperack P., Mercer J., Strobel M., Copeland N., Jenkins N. Retroviral sequences located within an intron of the dilute gene alter dilute expression in a tissue-specific manner. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2326–2332. doi: 10.1002/j.1460-2075.1995.tb07227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M., Block S., Spudich J. Myosin movement in vitroa quantitative assay using oriented actin cables from Nitella . Methods Enzymol. 1986;134:531–544. doi: 10.1016/0076-6879(86)34118-1. [DOI] [PubMed] [Google Scholar]
- Shima D., Haldar K., Pepperkok R., Watson R., Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D., Cabrera-Poch N., Pepperkok R., Warren G. An ordered inheritance strategy for the Golgi apparatusvisualization of mitotic disassembly reveals a role for the mitotic spindle. J. Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Shochet N., Blasberger D., Kashman Y. Latrunculinsnovel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Starobudov S., Golichenkov V. Pigment migration in dermal melanophores of amphibian larvae at the interphase and during mitosis. Ontogenz. 1988;19:279–283. [PubMed] [Google Scholar]
- Steffen W., Karki S., Vaughan K.T., Vallee R.B., Holzbaur E.L., Weiss D.G., Kuznetsov S.A. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheetsprocedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma M., Zill A., Le Bot N., Vernos I., Gelfand V. Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores. J. Cell Biol. 1998;143:1547–1558. doi: 10.1083/jcb.143.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu. Rev. Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Warren G., Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C., Sanger J.W., Sanger J.M. Dynamics of organelles in the mitotic spindles of living cellsmembrane and microtubule interactions. Cell Motil. Cytoskeleton. 1993;26:19–39. doi: 10.1002/cm.970260104. [DOI] [PubMed] [Google Scholar]
- Waters J., Salmon E. Pathways of spindle assembly. Curr. Opin. Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Weimer E., Wenzel T., Deerinck T., Ellisman M., Subramani S. Visualization of the peroxisomal compartment in living mammalian cellsdynamic behavior and association with microtubules. J. Cell Biol. 1997;136:71–80. doi: 10.1083/jcb.136.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Bowers B., Wei Q., Kocher B., Hammer J. Myosin V associates with melanosomes is mouse melanocytesevidence that myosin V is an organelle motor. J. Cell Sci. 1997;110:847–859. doi: 10.1242/jcs.110.7.847. [DOI] [PubMed] [Google Scholar]
- Wu X., Bowers B., Rao K., Wei Q., Hammer J. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo J. Cell Biol. 143 1998. 1899 1918a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kocher B., Wei Q., Hammer J.A., III. Myosin Va associates with microtubule-rich domains in both interphase and dividing cells Cell Motil. Cytoskeleton. 40 1998. 286 303b [DOI] [PubMed] [Google Scholar]