Abstract
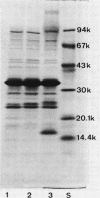
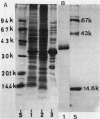
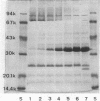
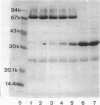
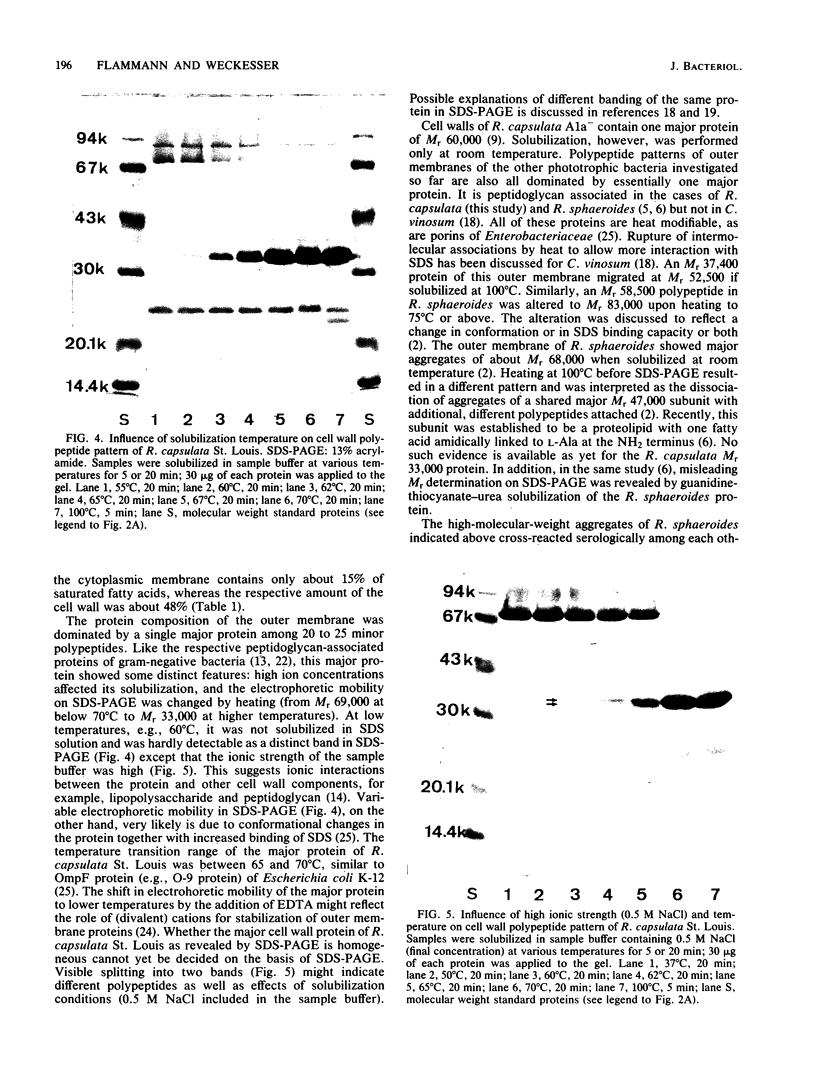
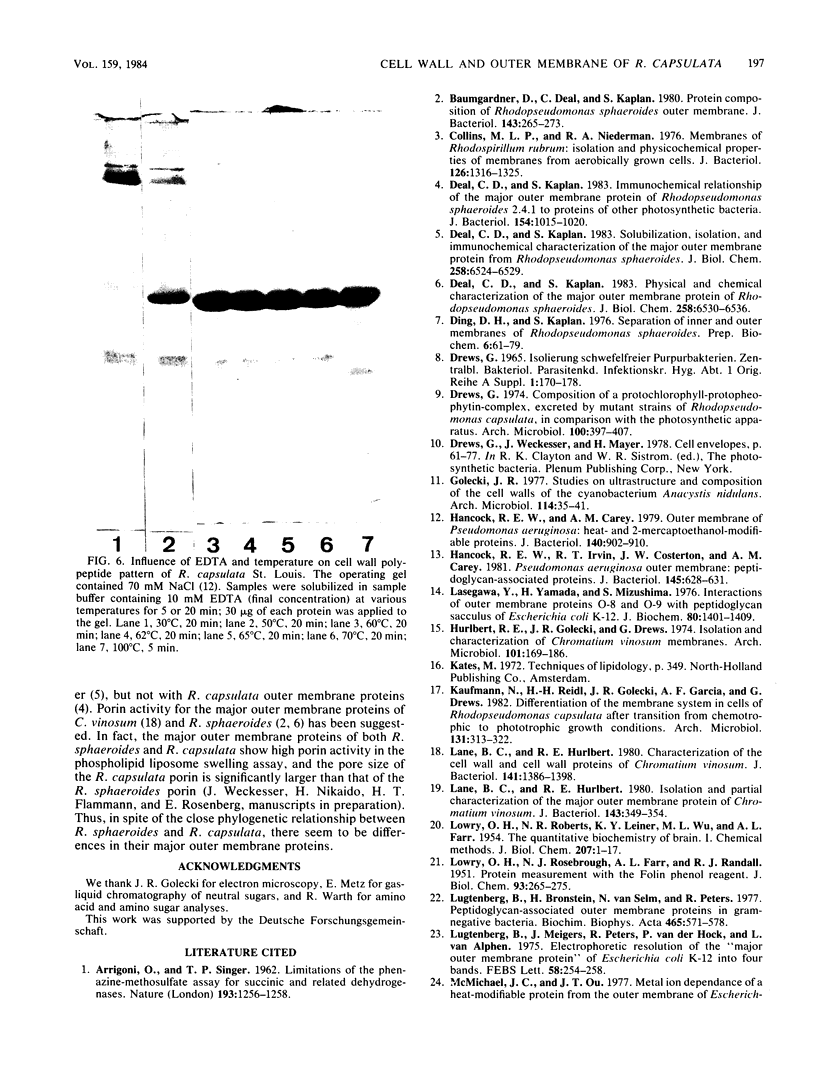
Sucrose density gradient centrifugation of cell envelopes of chemotrophically grown cells of Rhodopseudomonas capsulata St. Louis (= ATCC 23782) resulted in the separation of a cytoplasmic membrane from a cell wall fraction (buoyant densities, 1.139 and 1.215 g/cm3, respectively). The cell wall fractions (untreated or Triton extracted) contained peptidoglycan- and lipopolysaccharide-specific components. Their neutral sugar content, mainly rhamnose and galactose, was high (250 and 100 micrograms/mg [dry weight] of material) due to a non-lipopolysaccharide polymer. The fatty acid content was low (less than or equal to 60 micrograms/mg [dry weight] of material), and half of it was contributed by lipopolysaccharide (3-OH-C10:0, C12:1, and 3-oxo-C14:0). The predominant other fatty acid was C18:1. An outer membrane fraction, obtained by lysozyme treatment of the Triton-extracted cell wall, showed essentially the same chemical composition except for almost complete removal of peptidoglycan. Saline extraction (0.9% NaCl, 37 degrees C, 2 h) removed a lipopolysaccharide-protein(-phospholipid?) complex from whole cells of R. capsulata St. Louis. The polypeptide patterns of the cell wall and outer membrane as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis comprised 20 to 25 different polypeptides (most of them very faint) and were dominated by a single, heat-modifiable major protein (Mr 69,000 after solubilization below 60 degrees C; Mr 33,000 at temperatures above 70 degrees C).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Baumgardner D., Deal C., Kaplan S. Protein composition of Rhodopseudomonas sphaeroides outer membrane. J Bacteriol. 1980 Jul;143(1):265–273. doi: 10.1128/jb.143.1.265-273.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: isolation and physicochemical properties of membranes from aerobically grown cells. J Bacteriol. 1976 Jun;126(3):1316–1325. doi: 10.1128/jb.126.3.1316-1325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal C. D., Kaplan S. Immunochemical relationship of the major outer membrane protein of Rhodopseudomonas sphaeroides 2.4.1 to proteins of other photosynthetic bacteria. J Bacteriol. 1983 May;154(2):1015–1020. doi: 10.1128/jb.154.2.1015-1020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal C. D., Kaplan S. Physical and chemical characterization of the major outer membrane protein of Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6530–6536. [PubMed] [Google Scholar]
- Deal C. D., Kaplan S. Solubilization, isolation, and immunochemical characterization of the major outer membrane protein from Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6524–6529. [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Golecki J. R. Studies on ultrastructure and composition of cell walls of the cyanobacterium Anacystis nidulans. Arch Microbiol. 1977 Jul 26;114(1):35–41. doi: 10.1007/BF00429627. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Irvin R. T., Costerton J. W., Carey A. M. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981 Jan;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Hurlbert R. E. Isolation and characterization of Chromatium vinosum membranes. Arch Microbiol. 1974;101(2):169–186. doi: 10.1007/BF00455937. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane B. C., Hurlbert R. E. Characterization of the cell wall and cell wall proteins of Chromatium vinosum. J Bacteriol. 1980 Mar;141(3):1386–1398. doi: 10.1128/jb.141.3.1386-1398.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B. C., Hurlbert R. E. Isolation and partial characterization of the major outer membrane protein of Chromatium vinosum. J Bacteriol. 1980 Jul;143(1):349–354. doi: 10.1128/jb.143.1.349-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Metal ion dependence of a heat-modifiable protein from the outer membrane of Escherichia coli upon sodium dodecyl sulfate-gel electrophoresis. J Bacteriol. 1977 Oct;132(1):314–320. doi: 10.1128/jb.132.1.314-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oelze J., Golecki J. R., Kleinig H., Weckesser J. Characterization of two cell-envelope fractions from chemotrophically grown Rhodospirillum rubrum. Antonie Van Leeuwenhoek. 1975;41(3):273–286. doi: 10.1007/BF02565063. [DOI] [PubMed] [Google Scholar]
- Omar A. S., Flammann H. T., Borowiak D., Weckesser J. Lipopolysaccharides of two strains of the phototrophic bacterium Rhodopseudomonas capsulata. Arch Microbiol. 1983 Jun;134(3):212–216. doi: 10.1007/BF00407760. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Biedermann M., Drews G. Die Fettsäuren in ganzen Zellen, Thylakoiden und Lipopolysacchariden von Rhodospirillum rubrum und Rhodopseudomonas capsulata. Arch Mikrobiol. 1969;66(3):273–280. [PubMed] [Google Scholar]
- Solioz M., Marrs B. The gene transfer agent of Rhodopseudomonas capsulata. Purification and characterization of its nucleic acid. Arch Biochem Biophys. 1977 May;181(1):300–307. doi: 10.1016/0003-9861(77)90508-2. [DOI] [PubMed] [Google Scholar]
- Wall J. D., Weaver P. F., Gest H. Gene transfer agents, bacteriophages, and bacteriocins of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):217–224. doi: 10.1007/BF00447140. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Fromme I. Chemical analysis of and degradation studies on the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Mar;109(3):1106–1113. doi: 10.1128/jb.109.3.1106-1113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Ladwig R. Localization and biological and physicochemical properties of the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Apr;110(1):346–353. doi: 10.1128/jb.110.1.346-353.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Mayer H. Lipopolysaccharides of photosynthetic prokaryotes. Annu Rev Microbiol. 1979;33:215–239. doi: 10.1146/annurev.mi.33.100179.001243. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Mayer H., Drews G. The identification of 3-O-methyl-L-rhamnose (L-acofriose) as constituent of the lipopolysaccharide of Rhodopseudomonas capsulata. Eur J Biochem. 1970 Sep;16(1):158–160. doi: 10.1111/j.1432-1033.1970.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Wood B. J., Nichols B. W., James A. T. The lipids and fatty acid metabolism of photosynthetic bacteria. Biochim Biophys Acta. 1965 Oct 4;106(2):261–273. doi: 10.1016/0005-2760(65)90034-2. [DOI] [PubMed] [Google Scholar]