Abstract
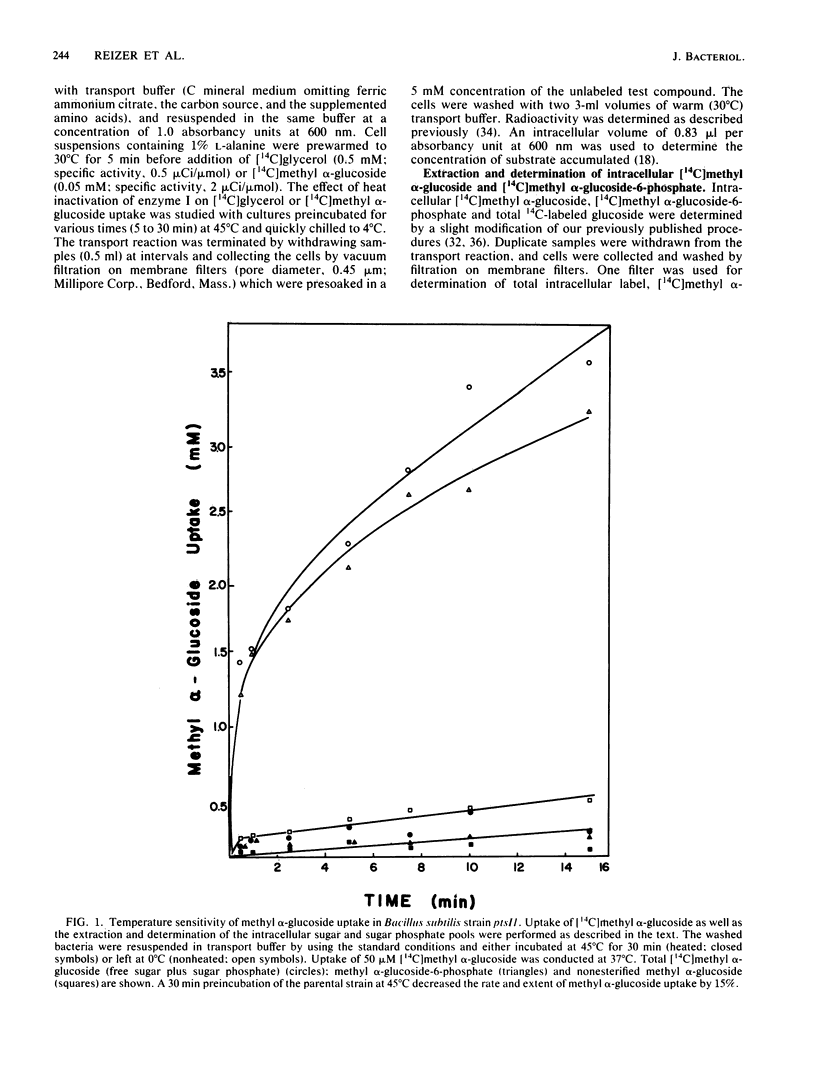
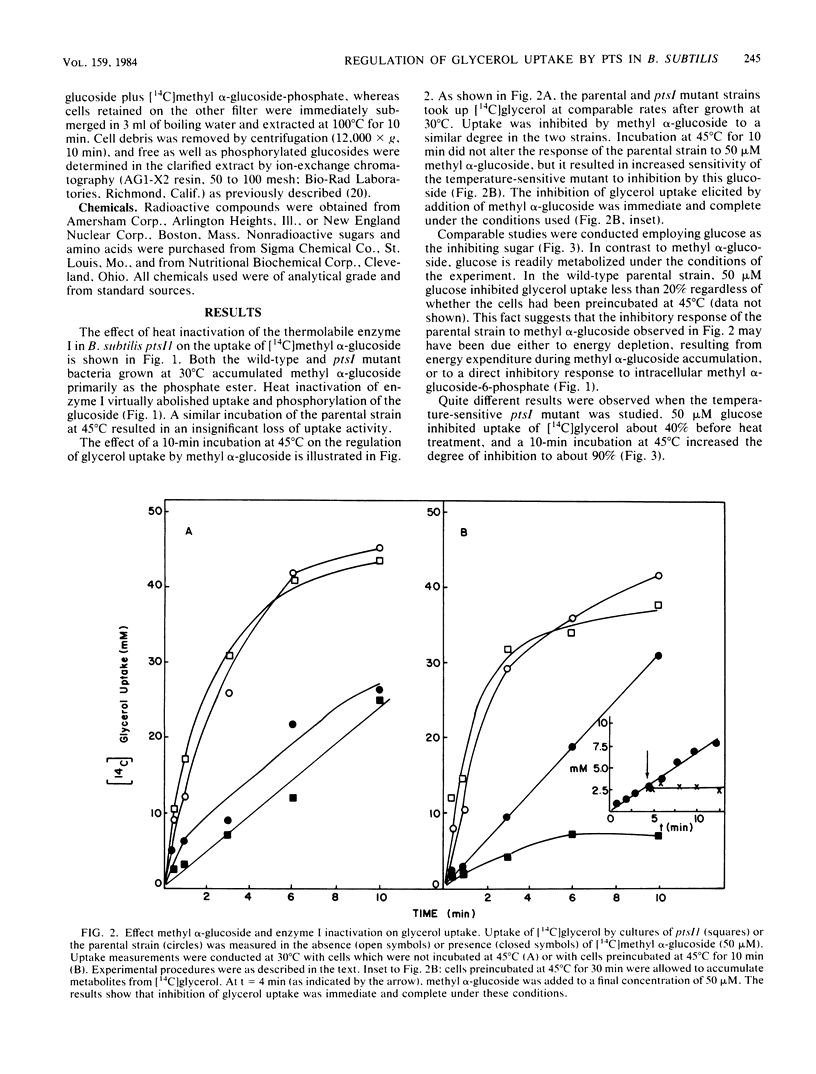
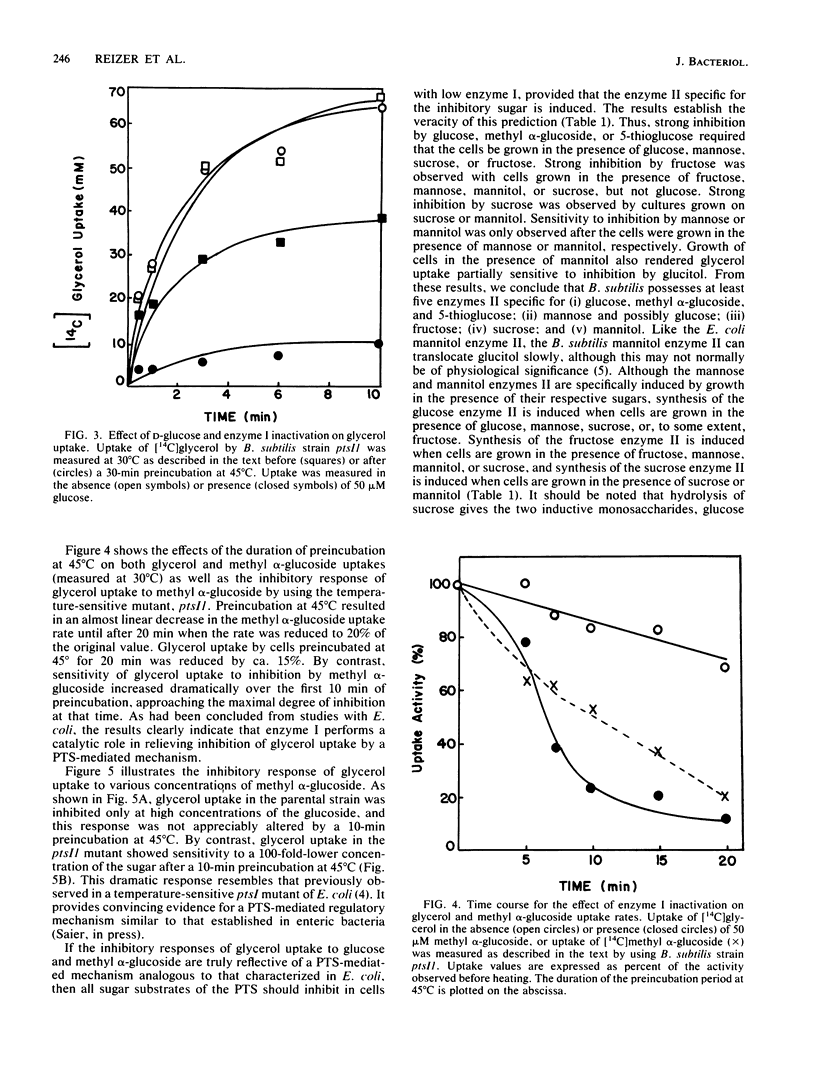
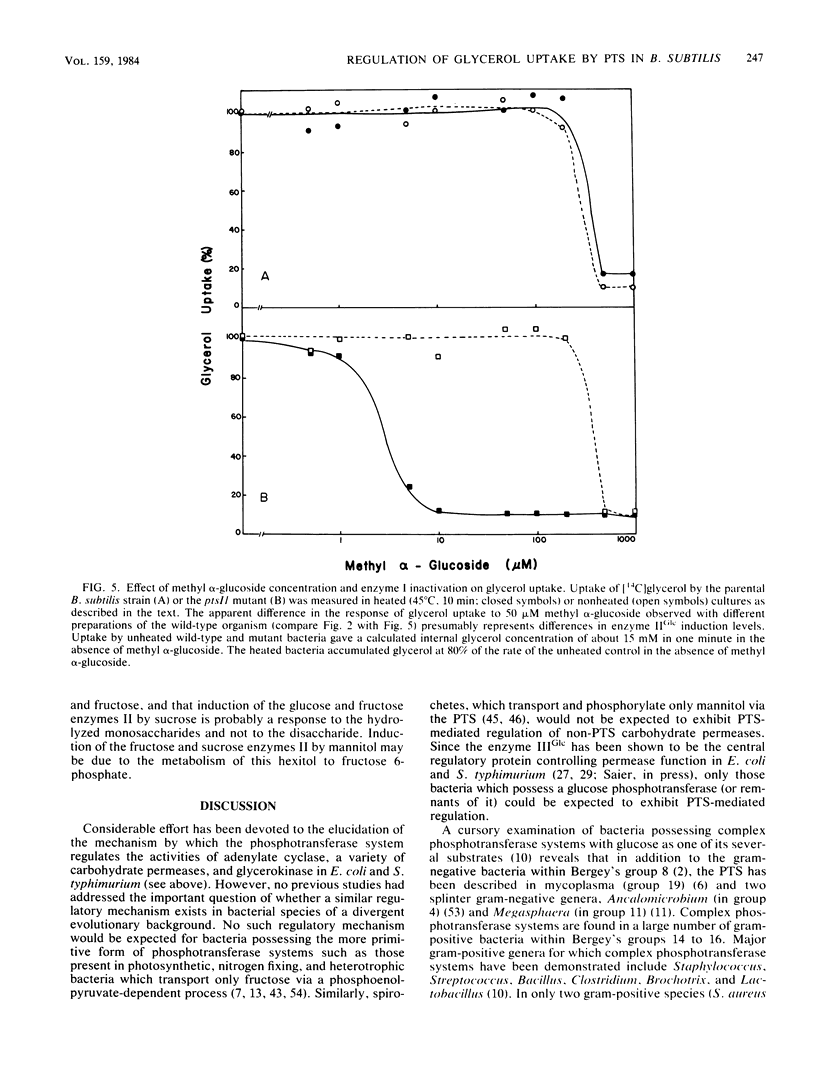
Enteric bacteria have been previously shown to regulate the uptake of certain carbohydrates (lactose, maltose, and glycerol) by an allosteric mechanism involving the catalytic activities of the phosphoenolpyruvate-sugar phosphotransferase system. In the present studies, a ptsI mutant of Bacillus subtilis, possessing a thermosensitive enzyme I of the phosphotransferase system, was used to gain evidence for a similar regulatory mechanism in a gram-positive bacterium. Thermoinactivation of enzyme I resulted in the loss of methyl alpha-glucoside uptake activity and enhanced sensitivity of glycerol uptake to inhibition by sugar substrates of the phosphotransferase system. The concentration of the inhibiting sugar which half maximally blocked glycerol uptake was directly related to residual enzyme I activity. Each sugar substrate of the phosphotransferase system inhibited glycerol uptake provided that the enzyme II specific for that sugar was induced to a sufficiently high level. The results support the conclusion that the phosphotransferase system regulates glycerol uptake in B. subtilis and perhaps in other gram-positive bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman M., Zwaig N., Lin E. C. Suppression of a pleiotropic mutant affecting glycerol dissimilation. Biochem Biophys Res Commun. 1970 Jan 23;38(2):272–278. doi: 10.1016/0006-291x(70)90708-4. [DOI] [PubMed] [Google Scholar]
- Button D. K., Egan J. B., Hengstenberg W., Morse M. L. Carbohydrate transport in Staphylococcus aureus. IV. Maltose accumulation and metabolism. Biochem Biophys Res Commun. 1973 Jun 8;52(3):850–855. doi: 10.1016/0006-291x(73)91015-2. [DOI] [PubMed] [Google Scholar]
- Castro L., Feucht B. U., Morse M. L., Saier M. H., Jr Regulation of carbohydrate permeases and adenylate cyclase in Escherichia coli. Studies with mutant strains in which enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system is thermolabile. J Biol Chem. 1976 Sep 25;251(18):5522–5527. [PubMed] [Google Scholar]
- Chalumeau H., Delobbe A., Gay P. Biochemical and genetic study of D-glucitol transport and catabolism in Bacillus subtilis. J Bacteriol. 1978 Jun;134(3):920–928. doi: 10.1128/jb.134.3.920-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P., Razin S. Distribution of a phosphoenolypyruvate-dependent sugar phosphotransferase system in mycoplasms. J Bacteriol. 1973 Jan;113(1):212–217. doi: 10.1128/jb.113.1.212-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Schlegel H. G. Different pathways for fructose and glucose utilization in Rhodopseudomonas capsulata and demonstration of 1-phosphofructokinase in phototrophic bacteria. Biochim Biophys Acta. 1974 Jul 17;358(1):221–225. doi: 10.1016/0005-2744(74)90273-3. [DOI] [PubMed] [Google Scholar]
- Delobbe A., Haguenauer R., Rapoport G. Studies on the transport of -methyl-D-glucoside in Bacillus subtilis 168. Biochimie. 1971;53(9):1015–1021. doi: 10.1016/s0300-9084(71)80069-x. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Lee C. A., Saier M. H., Jr Phosphoenolpyruvate-dependent sugar phosphotransferase activity in Megasphaera elsdenii. Can J Microbiol. 1981 Sep;27(9):949–952. doi: 10.1139/m81-148. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Schmidt M. R., Saier M. H., Jr Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):239–244. doi: 10.1002/jcb.1982.240180211. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):172–183. doi: 10.1016/0926-6585(65)90101-9. [DOI] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Freese E. Purification and properties of fructose-1,6-bisphosphatase of Bacillus subtilis. J Biol Chem. 1979 Jun 25;254(12):5340–5349. [PubMed] [Google Scholar]
- Gay P., Cordier P., Marquet M., Delobbe A. Carbohydrate metabolism and transport in Bacillus subtilis. A study of ctr mutations. Mol Gen Genet. 1973 Mar 19;121(4):355–368. doi: 10.1007/BF00433234. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lindgren V., Rutberg L. Glycerol metabolism in Bacillus subtilis: gene-enzyme relationships. J Bacteriol. 1974 Aug;119(2):431–442. doi: 10.1128/jb.119.2.431-442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. M., Thoms B. Role of sugar uptake and metabolic intermediates on catabolite repression in Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):217–224. doi: 10.1128/jb.129.1.217-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet M., Creignou M. C., Dedonder R. The phosphoenolpyruvate : methyl-alpha-D-glucoside phosphotransferase system in Bacillus subtilis Marburg 168 : purification and identification of the phosphocarrier protein (HPr). Biochimie. 1976;58(4):435–441. doi: 10.1016/s0300-9084(76)80254-4. [DOI] [PubMed] [Google Scholar]
- Marquet M., Creignou M. C., Dedonder R. The phosphoenolpyruvate : methyl-alpha-d-glucoside phosphotransferase system in Bacillus subtilis Marburg : kinetic studies of enzyme ii and evidence for a phosphoryl enzyme ii intermediate. Biochimie. 1978;60(11-12):1283–1287. doi: 10.1016/s0300-9084(79)80445-9. [DOI] [PubMed] [Google Scholar]
- Mindich L. Pathway for oxidative dissimilation of glycerol in Bacillur subtilis. J Bacteriol. 1968 Aug;96(2):565–566. doi: 10.1128/jb.96.2.565-566.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet B., Gay P., Dedonder R. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol Gen Genet. 1975;136(4):337–349. doi: 10.1007/BF00341718. [DOI] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal M., Kunst F., Lepesant J. A., Dedonder R. Characterization of two sucrase activities in Bacillus subtilis Marburg. Biochimie. 1971;53(10):1059–1066. doi: 10.1016/s0300-9084(71)80193-1. [DOI] [PubMed] [Google Scholar]
- Perret J., Gay P. Kinetic study of a phosphoryl exchange reaction between fructose and fructose 1-phosphate catalyzed by the membrane-bound enzyme II of the phosphoenolpyruvate-fructose 1-phosphotransferase system of Bacillus subtilis. Eur J Biochem. 1979 Dec;102(1):237–246. doi: 10.1111/j.1432-1033.1979.tb06285.x. [DOI] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Thalenfeld B., Grossowicz N. Methyl-alpha-D-glucoside uptake and splitting by a thermophilic bacillus. Nature. 1976 Apr 1;260(5550):433–435. doi: 10.1038/260433a0. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Phosphorylation of glycerol in Staphylococcus aureus. J Bacteriol. 1973 May;114(2):880–881. doi: 10.1128/jb.114.2.880-881.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheb S. A. Perméation du glycérol et sporulation chez Bacillus subtilis. Can J Microbiol. 1972 Aug;18(8):1307–1313. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Roseman S. Phosphoenolpyruvate-dependent fructose phosphorylation in photosynthetic bacteria. J Biol Chem. 1971 Dec 25;246(24):7819–7821. [PubMed] [Google Scholar]
- Saier M. H., Jr, Newman M. J. Direct transfer of the phosphoryl moiety of mannitol 1-phosphate to [14C]mannitol catalyzed by the enzyme II complexes of the phosphoenolpyruvate: mannitol phosphotransferase systems in Spirochaeta aurantia and Salmonella typhimurium. J Biol Chem. 1976 Jun 25;251(12):3834–3837. [PubMed] [Google Scholar]
- Saier M. H., Jr, Newman M. J., Rephaeli A. W. Properties of a phosphoenolpyruvate: mannitol phosphotransferase system in Spirochaeta aurantia. J Biol Chem. 1977 Dec 25;252(24):8890–8898. [PubMed] [Google Scholar]
- Saier M. H., Jr, Novotny M. J., Comeau-Fuhrman D., Osumi T., Desai J. D. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1983 Sep;155(3):1351–1357. doi: 10.1128/jb.155.3.1351-1357.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D. Regulation of carbohydrate uptake in gram-positive bacteria. J Biol Chem. 1976 Feb 10;251(3):893–894. [PubMed] [Google Scholar]
- Saier M. H., Jr, Staley J. T. Phosphoenolpyruvate:sugar phosphotransferase system in Ancalomicrobium adetum. J Bacteriol. 1977 Aug;131(2):716–718. doi: 10.1128/jb.131.2.716-718.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L. Pathways of D-fructose and D-glucose catabolism in marine species of Alcaligenes, Pseudomonas marina, and Alteromonas communis. Arch Microbiol. 1977 Mar 1;112(2):169–172. doi: 10.1007/BF00429331. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


