Abstract
When the "suicide" vector pSUP1011, which carries transposon Tn5 (Kmr), was introduced into Rhizobium japonicum USDA 110, kanamycin-resistant (Kmr) colonies were detected at a frequency (4.2 X 10-6) ca. 30 times greater than the spontaneous kanamycin resistance frequency (1.4 X 10-7). Ten thousand Kmr mutants were isolated and tested for nutritional auxotrophy. Auxotrophs were detected at a frequency of 0.5%. The following classes of auxotrophs were identified: adenine- (three), histidine- (three), glutamate- (five), adenine plus thiamine- (nine), uracil- (three), pantothenic acid- (one), tryptophan- (three), and methionine- (three). Mutants blocked in symbiotic nitrogen fixation (Fix-) were also identified at a frequency of 3%. The glutamate auxotrophs were studied in more detail, and all five showed an altered expression of nitrogenase activity in free-living cultures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cen Y., Bender G. L., Trinick M. J., Morrison N. A., Scott K. F., Gresshoff P. M., Shine J., Rolfe B. G. Transposon mutagenesis in rhizobia which can nodulate both legumes and the nonlegume parasponia. Appl Environ Microbiol. 1982 Jan;43(1):233–236. doi: 10.1128/aem.43.1.233-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
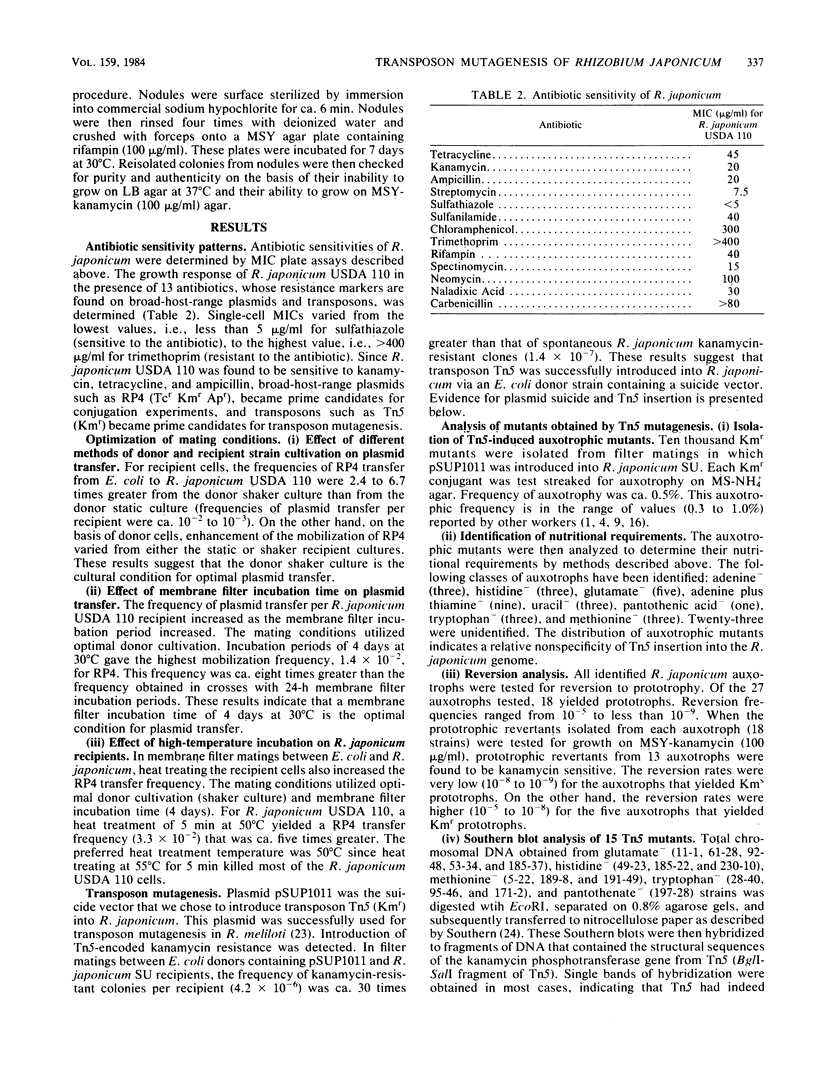
- Cole M. A., Elkan G. H. Multiple antibiotic resistance in Rhizobium japonicum. Appl Environ Microbiol. 1979 May;37(5):867–870. doi: 10.1128/aem.37.5.867-870.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Ely B., Croft R. H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982 Feb;149(2):620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D. Transfer of R factors to and between genetically marked sublines of Rhizobium japonicum. Appl Environ Microbiol. 1979 May;37(5):862–866. doi: 10.1128/aem.37.5.862-866.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Maier R. C., Norris H. J. Glassy cell carcinoma of the cervix. Obstet Gynecol. 1982 Aug;60(2):219–224. [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
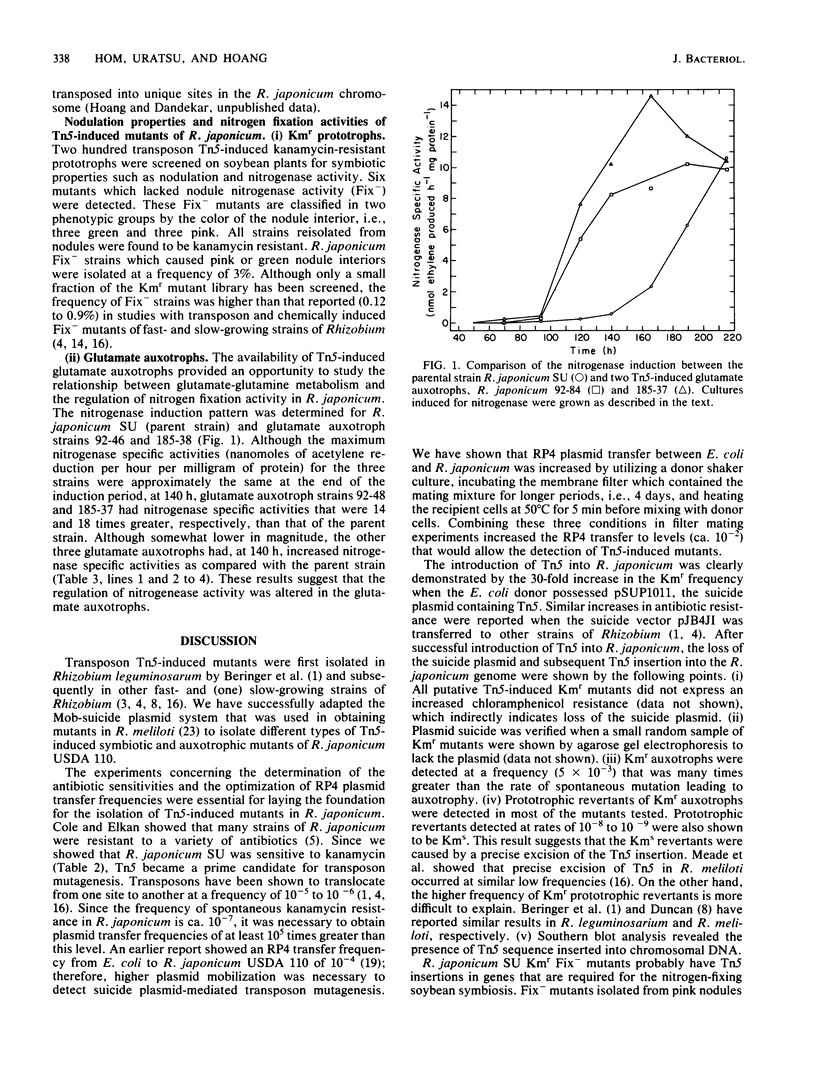
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Stacey G., Tandon S. R., Silver L. E., Brill W. J. Rhizobium japonicum mutants defective in symbiotic nitrogen fixation. J Bacteriol. 1982 Oct;152(1):485–494. doi: 10.1128/jb.152.1.485-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam K. T., Chan I., Morandi C. Regulation of nitrogen fixation. Nitrogenase-derepressed mutants of Klebsiella pneumoniae. Biochim Biophys Acta. 1975 Nov 11;408(2):101–111. doi: 10.1016/0005-2728(75)90002-x. [DOI] [PubMed] [Google Scholar]
- Shanmugam K. T., Valentine R. C. Microbial production of ammonium ion from nitrogen. Proc Natl Acad Sci U S A. 1975 Jan;72(1):136–139. doi: 10.1073/pnas.72.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S. Glutamine synthetase and ammonium regulation of nitrogenase synthesis in Klebsiella. Nature. 1974 Oct 11;251(5475):481–485. doi: 10.1038/251481a0. [DOI] [PubMed] [Google Scholar]


